Redundant catalases detoxify phagocyte reactive oxygen and facilitate Histoplasma capsulatum pathogenesis
- PMID: 23589579
- PMCID: PMC3697612
- DOI: 10.1128/IAI.00173-13
Redundant catalases detoxify phagocyte reactive oxygen and facilitate Histoplasma capsulatum pathogenesis
Abstract
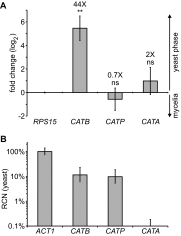
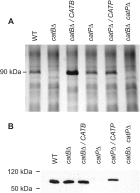
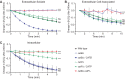
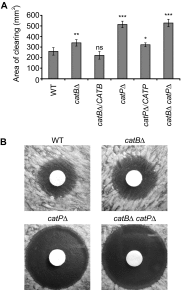
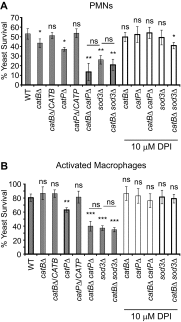
Histoplasma capsulatum is a respiratory pathogen that infects phagocytic cells. The mechanisms allowing Histoplasma to overcome toxic reactive oxygen molecules produced by the innate immune system are an integral part of Histoplasma's ability to survive during infection. To probe the contribution of Histoplasma catalases in oxidative stress defense, we created and analyzed the virulence defects of mutants lacking CatB and CatP, which are responsible for extracellular and intracellular catalase activities, respectively. Both CatB and CatP protected Histoplasma from peroxide challenge in vitro and from antimicrobial reactive oxygen produced by human neutrophils and activated macrophages. Optimal protection required both catalases, as the survival of a double mutant lacking both CatB and CatP was lower than that of single-catalase-deficient cells. Although CatB contributed to reactive oxygen species defenses in vitro, CatB was dispensable for lung infection and extrapulmonary dissemination in vivo. Loss of CatB from a strain also lacking superoxide dismutase (Sod3) did not further reduce the survival of Histoplasma yeasts. Nevertheless, some catalase function was required for pathogenesis since simultaneous loss of both CatB and CatP attenuated Histoplasma virulence in vivo. These results demonstrate that Histoplasma's dual catalases comprise a system that enables Histoplasma to efficiently overcome the reactive oxygen produced by the innate immune system.
Figures


References
-
- DeLeo FR, Allen LA, Apicella M, Nauseef WM. 1999. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163:6732–6740 - PubMed
-
- Cohen MS, Isturiz RE, Malech HL, Root RK, Wilfert CM, Gutman L, Buckley RH. 1981. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am. J. Med. 71:59–66 - PubMed
-
- Soler-Palacin P, Margareto C, Llobet P, Asensio O, Hernandez M, Caragol I, Espanol T. 2007. Chronic granulomatous disease in pediatric patients: 25 years of experience. Allergol. Immunopathol. (Madr.) 35:83–89 - PubMed
-
- Fairfield AS, Abosch A, Ranz A, Eaton JW, Meshnick SR. 1988. Oxidant defense enzymes of Plasmodium falciparum. Mol. Biochem. Parasitol. 30:77–82 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

