The survival of memory CD8 T cells that is mediated by IL-15 correlates with sustained protection against malaria
- PMID: 23589611
- PMCID: PMC3646969
- DOI: 10.4049/jimmunol.1203396
The survival of memory CD8 T cells that is mediated by IL-15 correlates with sustained protection against malaria
Abstract
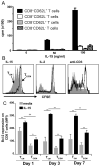
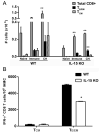
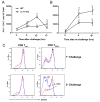
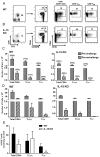
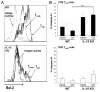
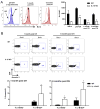
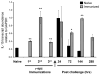
Ag-specific memory T cell responses elicited by infections or vaccinations are inextricably linked to long-lasting protective immunity. Studies of protective immunity among residents of malaria endemic areas indicate that memory responses to Plasmodium Ags are not adequately developed or maintained, as people who survive episodes of childhood malaria are still vulnerable to either persistent or intermittent malaria infections. In contrast, multiple exposures to radiation-attenuated Plasmodium berghei sporozoites (Pb γ-spz) induce long-lasting protective immunity to experimental sporozoite challenge. We previously demonstrated that sterile protection induced by Pb γ-spz is MHC class I-dependent and CD8 T cells are the key effectors. IFN-γ(+) CD8 T cells that arise in Pb γ-spz-immunized B6 mice are found predominantly in the liver and are sensitive to levels of liver-stage Ag depot and they express CD44(hi)CD62L(lo) markers indicative of effector/effector memory phenotype. The developmentally related central memory CD8 T (TCM) cells express elevated levels of CD122 (IL-15Rβ), which suggests that CD8 TCM cells depend on IL-15 for maintenance. Using IL-15-deficient mice, we demonstrate in this study that although protective immunity is inducible in these mice, protection is short-lived, mainly owing to the inability of CD8 TCM cells to survive in the IL-15-deficient milieu. We present a hypothesis consistent with a model whereby intrahepatic CD8 TCM cells, being maintained by IL-15-mediated survival and basal proliferation, are conscripted into the CD8 effector/effector memory T cell pool during subsequent infections.
Figures

Similar articles
-
Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells.J Immunol. 2003 Aug 15;171(4):2024-34. doi: 10.4049/jimmunol.171.4.2024. J Immunol. 2003. PMID: 12902507
-
Memory CD8 T cells specific for plasmodia liver-stage antigens maintain protracted protection against malaria.Front Immunol. 2012 Dec 10;3:370. doi: 10.3389/fimmu.2012.00370. eCollection 2012. Front Immunol. 2012. PMID: 23233854 Free PMC article.
-
Memory phenotype CD8(+) T cells persist in livers of mice protected against malaria by immunization with attenuated Plasmodium berghei sporozoites.Eur J Immunol. 1999 Dec;29(12):3978-86. doi: 10.1002/(SICI)1521-4141(199912)29:12<3978::AID-IMMU3978>3.0.CO;2-0. Eur J Immunol. 1999. PMID: 10602007
-
The role of intrahepatic lymphocytes in mediating protective immunity induced by attenuated Plasmodium berghei sporozoites.Immunol Rev. 2000 Apr;174:123-34. doi: 10.1034/j.1600-0528.2002.00013h.x. Immunol Rev. 2000. PMID: 10807512 Review.
-
Memory T cells maintain protracted protection against malaria.Immunol Lett. 2014 Oct;161(2):189-95. doi: 10.1016/j.imlet.2014.03.011. Epub 2014 Apr 5. Immunol Lett. 2014. PMID: 24709142 Free PMC article. Review.
Cited by
-
From the draining lymph node to the liver: the induction and effector mechanisms of malaria-specific CD8+ T cells.Semin Immunopathol. 2015 May;37(3):211-20. doi: 10.1007/s00281-015-0479-3. Epub 2015 Apr 28. Semin Immunopathol. 2015. PMID: 25917387 Free PMC article. Review.
-
Liver-Stage Specific Response among Endemic Populations: Diet and Immunity.Front Immunol. 2015 Mar 20;6:125. doi: 10.3389/fimmu.2015.00125. eCollection 2015. Front Immunol. 2015. PMID: 25852693 Free PMC article. Review.
-
Imaging Plasmodium immunobiology in the liver, brain, and lung.Parasitol Int. 2014 Feb;63(1):171-86. doi: 10.1016/j.parint.2013.09.013. Epub 2013 Sep 27. Parasitol Int. 2014. PMID: 24076429 Free PMC article. Review.
-
Early effector cells survive the contraction phase in malaria infection and generate both central and effector memory T cells.J Immunol. 2015 Jun 1;194(11):5346-54. doi: 10.4049/jimmunol.1403216. Epub 2015 Apr 24. J Immunol. 2015. PMID: 25911759 Free PMC article.
-
Expanding CAR T cells in human platelet lysate renders T cells with in vivo longevity.J Immunother Cancer. 2019 Nov 28;7(1):330. doi: 10.1186/s40425-019-0804-9. J Immunother Cancer. 2019. PMID: 31779709 Free PMC article.
References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
-
- Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286:1377–1381. - PubMed
-
- Berenzon D, Schwenk RJ, Letellier L, Guebre-Xabier M, Williams J, Krzych U. Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells. J Immunol. 2003;171:2024–2034. - PubMed
-
- Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

