Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells
- PMID: 23589617
- PMCID: PMC3729324
- DOI: 10.4049/jimmunol.1202986
Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells
Abstract
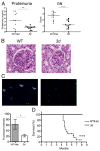
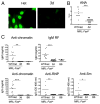
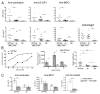
Nucleic acid (NA)-sensing TLRs (NA-TLRs) promote the induction of anti-nuclear Abs in systemic lupus erythematosus. However, the extent to which other nonnuclear pathogenic autoantibody specificities that occur in lupus and independently in other autoimmune diseases depend on NA-TLRs, and which immune cells require NA-TLRs in systemic autoimmunity, remains to be determined. Using Unc93b1(3d) lupus-prone mice that lack NA-TLR signaling, we found that all pathogenic nonnuclear autoantibody specificities examined, even anti-RBC, required NA-TLRs. Furthermore, we document that NA-TLRs in B cells were required for the development of antichromatin and rheumatoid factor. These findings support a unifying NA-TLR-mediated mechanism of autoantibody production that has both pathophysiological and therapeutic implications for systemic lupus erythematosus and several other humoral-mediated autoimmune diseases. In particular, our findings suggest that targeting of NA-TLR signaling in B cells alone would be sufficient to specifically block production of a broad diversity of autoantibodies.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures




Similar articles
-
Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation.J Immunol. 2014 May 15;192(10):4525-32. doi: 10.4049/jimmunol.1400098. Epub 2014 Apr 7. J Immunol. 2014. PMID: 24711620 Free PMC article.
-
Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus.Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):12061-6. doi: 10.1073/pnas.0905441106. Epub 2009 Jul 2. Proc Natl Acad Sci U S A. 2009. PMID: 19574451 Free PMC article.
-
Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing.J Exp Med. 2009 Jun 8;206(6):1339-50. doi: 10.1084/jem.20082316. Epub 2009 May 18. J Exp Med. 2009. PMID: 19451267 Free PMC article.
-
The role of toll-like receptors in systemic lupus erythematosus.Springer Semin Immunopathol. 2006 Oct;28(2):131-43. doi: 10.1007/s00281-006-0034-3. Epub 2006 Sep 19. Springer Semin Immunopathol. 2006. PMID: 17047954 Review.
-
Toll-like receptor signalling in B cells during systemic lupus erythematosus.Nat Rev Rheumatol. 2021 Feb;17(2):98-108. doi: 10.1038/s41584-020-00544-4. Epub 2020 Dec 18. Nat Rev Rheumatol. 2021. PMID: 33339987 Free PMC article. Review.
Cited by
-
Toll-like receptors and chronic inflammation in rheumatic diseases: new developments.Nat Rev Rheumatol. 2016 Jun;12(6):344-57. doi: 10.1038/nrrheum.2016.61. Epub 2016 May 12. Nat Rev Rheumatol. 2016. PMID: 27170508 Review.
-
Toll-like receptors 7 and 9 regulate the proliferation and differentiation of B cells in systemic lupus erythematosus.Front Immunol. 2023 Feb 15;14:1093208. doi: 10.3389/fimmu.2023.1093208. eCollection 2023. Front Immunol. 2023. PMID: 36875095 Free PMC article. Review.
-
The Role of Exposomes in the Pathophysiology of Autoimmune Diseases I: Toxic Chemicals and Food.Pathophysiology. 2021 Dec 18;28(4):513-543. doi: 10.3390/pathophysiology28040034. Pathophysiology. 2021. PMID: 35366249 Free PMC article. Review.
-
Functional Characterization of CD11c+ Age-Associated B Cells as Memory B Cells.J Immunol. 2019 Dec 1;203(11):2817-2826. doi: 10.4049/jimmunol.1900404. Epub 2019 Oct 21. J Immunol. 2019. PMID: 31636237 Free PMC article.
-
Neurotoxic or Neuroprotective? Current Controversies in SCI-Induced Autoimmunity.Curr Phys Med Rehabil Rep. 2013 Sep;1(3):10.1007/s40141-013-0021-2. doi: 10.1007/s40141-013-0021-2. Curr Phys Med Rehabil Rep. 2013. PMID: 24416711 Free PMC article.
References
-
- Izui S, Ibnou-Zekri N, Fossati-Jimack L, Iwamoto M. Lessons from BXSB and related mouse models. Int Rev Immunol. 2000;19:447–472. - PubMed
-
- Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

