Butyrophilin Btn2a2 inhibits TCR activation and phosphatidylinositol 3-kinase/Akt pathway signaling and induces Foxp3 expression in T lymphocytes
- PMID: 23589618
- PMCID: PMC3736090
- DOI: 10.4049/jimmunol.1203325
Butyrophilin Btn2a2 inhibits TCR activation and phosphatidylinositol 3-kinase/Akt pathway signaling and induces Foxp3 expression in T lymphocytes
Abstract
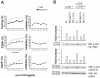
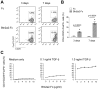
The butyrophilin-related protein Btn2a2 was upregulated on murine APC including CD19(+) B cells, CD11b(+)F4/80(+) peritoneal macrophages, and CD11c(+) bone marrow-derived dendritic cells after activation with LPS or Pam3CysK4, suggesting a role in modulation of T lymphocytes. Consistent with this, binding of mouse Btn2a2-Fc to CD3(+) primary mouse T cells stimulated with anti-CD3 and anti-CD28 reduced the number of proliferating cells and entry of cells into the cell cycle. Binding of Btn2a2-Fc to anti-CD3-stimulated T cells inhibited CD3ε, Zap70, and subsequent Erk1/2 activation. It also interfered with activation of the regulatory subunit of PI3K, p85, and activation of Akt in T cells stimulated with both anti-CD3 and anti-CD28. Inhibition of Akt activation by Btn2a2-Fc was, in contrast to inhibition by programmed death ligand-1-Fc, not overcome by anti-CD28 costimulation. Using Foxp3-GFP-transgenic, naive T cells, Btn2a2-Fc induced de novo expression of Foxp3 in a dose-dependent manner, and Btn2a2-Fc-induced CD4(+)CD25(+)Foxp3(+) T cells had inhibitory properties. The data indicate an important physiological role for Btn2a2 in inhibiting T cell activation and inducing Foxp3(+) regulatory T cells.
Figures



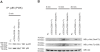

References
-
- Heid HW, Winter S, Bruder G, Keenan TW, Jarasch ED. Butyrophilin, an apical plasma membrane-associated glycoprotein characteristic of lactating mammary glands of diverse species. Biochim Biophys Acta. 1983;728:228–238. - PubMed
-
- Vernet C, Boretto J, Mattéi MG, Takahashi M, Jack LJ, Mather IH, Rouquier S, Pontarotti P. Evolutionary study of multigenic families mapping close to the human MHC class I region. J Mol Evol. 1993;37:600–612. - PubMed
-
- Rhodes DA, Stammers M, Malcherek G, Beck S, Trowsdale J. The cluster of BTN genes in the extended major histocompatibility complex. Genomics. 2001;71:351–362. - PubMed
-
- Tazi-Ahnini R, Henry J, Offer C, Bouissou-Bouchouata C, Mather IH, Pontarotti P. Cloning, localization, and structure of new members of the butyrophilin gene family in the juxta-telomeric region of the major histocompatibility complex. Immunogenetics. 1997;47:55–63. - PubMed
-
- Stammers M, Rowen L, Rhodes D, Trowsdale J, Beck S. BTL-II: a polymorphic locus with homology to the butyrophilin gene family, located at the border of the major histocompatibility complex class II and class III regions in human and mouse. Immunogenetics. 2000;51:373–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

