The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial
- PMID: 23589670
- PMCID: PMC3685899
- DOI: 10.1182/blood-2012-06-436345
The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial
Abstract
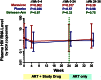
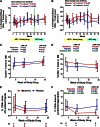
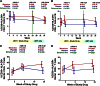
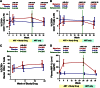
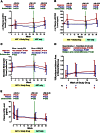
The CCR5 inhibitor maraviroc has been hypothesized to decrease T-cell activation in HIV-infected individuals, but its independent immunologic effects have not been established in a placebo-controlled trial. We randomized 45 HIV-infected subjects with CD4 counts <350 cells per mm(3) and plasma HIV RNA levels <48 copies per mL on antiretroviral therapy (ART) to add maraviroc vs placebo to their regimen for 24 weeks followed by 12 weeks on ART alone. Compared with placebo-treated subjects, maraviroc-treated subjects unexpectedly experienced a greater median increase in % CD38+HLA-DR+ peripheral blood CD8+ T cells at week 24 (+2.2% vs -0.7%, P = .014), and less of a decline in activated CD4+ T cells (P < .001). The % CD38+HLA-DR+ CD4+ and CD8+ T cells increased nearly twofold in rectal tissue (both P < .001), and plasma CC chemokine receptor type 5 (CCR5) ligand (macrophage-inflammatory protein 1β) levels increased 2.4-fold during maraviroc intensification (P < .001). During maraviroc intensification, plasma lipopolysaccharide declined, whereas sCD14 levels and neutrophils tended to increase in blood and rectal tissue. Although the mechanisms explaining these findings remain unclear, CCR5 ligand-mediated activation of T cells, macrophages, and neutrophils via alternative chemokine receptors should be explored. These results may have relevance for trials of maraviroc for HIV preexposure prophylaxis and graft-versus-host disease. This trial was registered at www.clinicaltrials.gov as #NCT00735072.
Figures
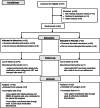

Comment in
-
Maraviroc-induced decrease in circulating bacterial products is not linked to an increase in immune activation in HIV-infected individuals.Blood. 2013 Sep 26;122(13):2282-3. doi: 10.1182/blood-2013-06-507012. Blood. 2013. PMID: 24072848 No abstract available.
-
Response: Maraviroc intensification and microbial translocation.Blood. 2013 Sep 26;122(13):2283-4. doi: 10.1182/blood-2013-08-516930. Blood. 2013. PMID: 24072849 No abstract available.
Similar articles
-
Maraviroc Intensification of cART in Patients with Suboptimal Immunological Recovery: A 48-Week, Placebo-Controlled Randomized Trial.PLoS One. 2015 Jul 24;10(7):e0132430. doi: 10.1371/journal.pone.0132430. eCollection 2015. PLoS One. 2015. PMID: 26208341 Free PMC article. Clinical Trial.
-
Maraviroc as intensification strategy in HIV-1 positive patients with deficient immunological response: an Italian randomized clinical trial.PLoS One. 2013 Nov 14;8(11):e80157. doi: 10.1371/journal.pone.0080157. eCollection 2013. PLoS One. 2013. PMID: 24244635 Free PMC article. Clinical Trial.
-
Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected.PLoS One. 2011;6(12):e27864. doi: 10.1371/journal.pone.0027864. Epub 2011 Dec 8. PLoS One. 2011. PMID: 22174752 Free PMC article. Clinical Trial.
-
Clinical utility of maraviroc.Clin Drug Investig. 2011;31(8):527-542. doi: 10.2165/11590700-000000000-00000. Clin Drug Investig. 2011. PMID: 21595497 Review.
-
Maraviroc: a review of its use in the management of CCR5-tropic HIV-1 infection.Drugs. 2010 Jun 18;70(9):1189-213. doi: 10.2165/11203940-000000000-00000. Drugs. 2010. PMID: 20518583 Review.
Cited by
-
Pathogenesis of Aging and Age-related Comorbidities in People with HIV: Highlights from the HIV ACTION Workshop.Pathog Immun. 2020 Jun 17;5(1):143-174. doi: 10.20411/pai.v5i1.365. eCollection 2020. Pathog Immun. 2020. PMID: 32856008 Free PMC article.
-
Probing the Interface of HIV and Inflammaging.Curr HIV/AIDS Rep. 2021 Jun;18(3):198-210. doi: 10.1007/s11904-021-00547-0. Epub 2021 Mar 11. Curr HIV/AIDS Rep. 2021. PMID: 33709322 Review.
-
Maraviroc Reduces Arterial Stiffness in PI-Treated HIV-infected Patients.Sci Rep. 2016 Jun 29;6:28853. doi: 10.1038/srep28853. Sci Rep. 2016. PMID: 27352838 Free PMC article.
-
Influence of immune activation and inflammatory response on cardiovascular risk associated with the human immunodeficiency virus.Vasc Health Risk Manag. 2015 Jan 6;11:35-48. doi: 10.2147/VHRM.S65885. eCollection 2015. Vasc Health Risk Manag. 2015. PMID: 25609975 Free PMC article. Review.
-
Antiretroviral therapy in HIV-1-infected individuals with CD4 count below 100 cells/mm3 results in differential recovery of monocyte activation.J Leukoc Biol. 2016 Jul;100(1):223-31. doi: 10.1189/jlb.5AB0915-406R. Epub 2015 Nov 25. J Leukoc Biol. 2016. PMID: 26609048 Free PMC article.
References
-
- Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. - PubMed
-
- Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146(2):87–95. - PubMed
-
- Lewden C, Chene G, Morlat P, et al. Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO8 APROCO-COPILOTE Study Group; Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) CO3 AQUITAINE Study Group. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46(1):72–77. - PubMed
-
- Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–1543. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AI36219/AI/NIAID NIH HHS/United States
- K23 CA157929/CA/NCI NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- P30AI08215/AI/NIAID NIH HHS/United States
- AI087035/AI/NIAID NIH HHS/United States
- P01 AI076174/AI/NIAID NIH HHS/United States
- R21 AI087035/AI/NIAID NIH HHS/United States
- CA157929/CA/NCI NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- R01 AI057020/AI/NIAID NIH HHS/United States
- P30 AI27763/AI/NIAID NIH HHS/United States
- AI057020/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- K99 HL108743/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

