Tobacco mosaic virus rods and spheres as supramolecular high-relaxivity MRI contrast agents
- PMID: 23589767
- PMCID: PMC3624747
- DOI: 10.1039/C3TB00461A
Tobacco mosaic virus rods and spheres as supramolecular high-relaxivity MRI contrast agents
Abstract
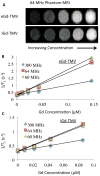
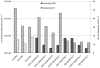
To compensate for the low sensitivity of magnetic resonance imaging (MRI), nanoparticles have been developed to deliver high payloads of contrast agents to sites of disease. Here, we report the development of supramolecular MRI contrast agents using the plant viral nanoparticle tobacco mosaic virus (TMV). Rod-shaped TMV nanoparticles measuring 300×18 nm were loaded with up to 3,500 or 2,000 chelated paramagnetic gadolinium (III) ions selectively at the interior (iGd-TMV) or exterior (eGd-TMV) surface, respectively. Spatial control is achieved through targeting either tyrosine or carboxylic acid side chains on the solvent exposed exterior or interior TMV surface. The ionic T1 relaxivity per Gd ion (at 60 MHz) increases from 4.9 mM-1s-1 for free Gd(DOTA) to 18.4 mM-1s-1 for eGd-TMV and 10.7 mM-1s-1 for iGd-TMV. This equates to T1 values of ~ 30,000 mM-1s-1 and ~ 35,000 mM-1s-1 per eGd-TMV and iGd-TMV nanoparticle. Further, we show that interior-labeled TMV rods can undergo thermal transition to form 170 nm-sized spherical nanoparticles containing ~ 25,000 Gd chelates and a per particle relaxivity of almost 400,000 mM-1s-1 (15.2 mM-1s-1 per Gd). This work lays the foundation for the use of TMV as a contrast agent for MRI.
Keywords: contrast agent; magnetic resonance imaging; tobacco mosaic virus; viral nanoparticle.
Figures


References
-
- Yan GP, Robinson L, Hogg P. Magnetic resonance imaging contrast agents: Overview and perspectives. Radiography. 2007;13:e5–e19.
-
- Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chemical Society Reviews. 2006;35:512. - PubMed
-
- Waters EA, Wickline SA. Contrast agents for MRI. Basic Research in Cardiology. 2008;103:114–121. - PubMed
-
- Floyd WC, et al. Conjugation Effects of Various Linkers on Gd(III) MRI Contrast Agents with Dendrimers: Optimizing the Hydroxypyridinonate (HOPO) Ligands with Nontoxic, Degradable Esteramide (EA) Dendrimers for High Relaxivity. Journal of the American Chemical Society. 2011;133:2390–2393. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

