The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters
- PMID: 23589833
- PMCID: PMC3768104
- DOI: 10.1152/physrev.00023.2012
The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters
Abstract
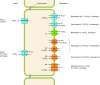
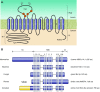
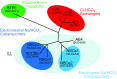
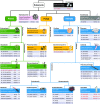
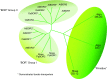
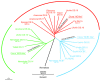
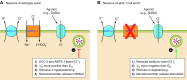
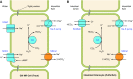
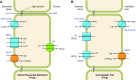
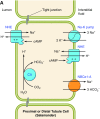
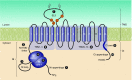
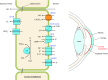
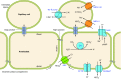
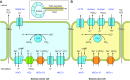
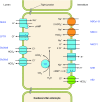
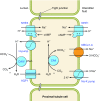
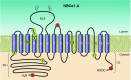
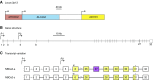
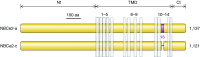
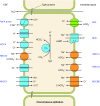
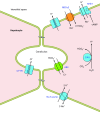
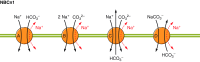
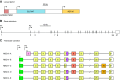
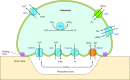
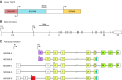
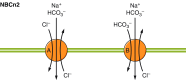
The mammalian Slc4 (Solute carrier 4) family of transporters is a functionally diverse group of 10 multi-spanning membrane proteins that includes three Cl-HCO3 exchangers (AE1-3), five Na(+)-coupled HCO3(-) transporters (NCBTs), and two other unusual members (AE4, BTR1). In this review, we mainly focus on the five mammalian NCBTs-NBCe1, NBCe2, NBCn1, NDCBE, and NBCn2. Each plays a specialized role in maintaining intracellular pH and, by contributing to the movement of HCO3(-) across epithelia, in maintaining whole-body pH and otherwise contributing to epithelial transport. Disruptions involving NCBT genes are linked to blindness, deafness, proximal renal tubular acidosis, mental retardation, and epilepsy. We also review AE1-3, AE4, and BTR1, addressing their relevance to the study of NCBTs. This review draws together recent advances in our understanding of the phylogenetic origins and physiological relevance of NCBTs and their progenitors. Underlying these advances is progress in such diverse disciplines as physiology, molecular biology, genetics, immunocytochemistry, proteomics, and structural biology. This review highlights the key similarities and differences between individual NCBTs and the genes that encode them and also clarifies the sometimes confusing NCBT nomenclature.
Figures














References
-
- Normal Table of Xenopus laevis (Daudin) (2nd ed.). New York: Garland, 1994.
-
- Abdulnour-Nakhoul S, Khuri RN, Nakhoul NL. Effect of norepinephrine on intracellular pH in kidney proximal tubule: role of Na+-(HCO3−)n cotransport. Am J Physiol Renal Physiol 275: F33–F45, 1998. - PubMed
-
- Abdulnour-Nakhoul S, Nakhoul NL, Wheeler SA, Wang P, Swenson ER, Orlando RC. HCO3− secretion in the esophageal submucosal glands. Am J Physiol Gastrointest Liver Physiol 288: G736–G744, 2005. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 NS018400/NS/NINDS NIH HHS/United States
- P01 HD032573/HD/NICHD NIH HHS/United States
- NS18400/NS/NINDS NIH HHS/United States
- HL090969/HL/NHLBI NIH HHS/United States
- R21 EY021646/EY/NEI NIH HHS/United States
- HD032573/HD/NICHD NIH HHS/United States
- DK30344/DK/NIDDK NIH HHS/United States
- R01 HL090969/HL/NHLBI NIH HHS/United States
- R01 DK030344/DK/NIDDK NIH HHS/United States
- EY021646/EY/NEI NIH HHS/United States
- DK81567/DK/NIDDK NIH HHS/United States
- R01 DK081567/DK/NIDDK NIH HHS/United States
- R37 DK030344/DK/NIDDK NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

