LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus
- PMID: 23589850
- PMCID: PMC3645523
- DOI: 10.1073/pnas.1214441110
LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus
Abstract
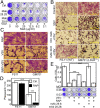
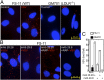
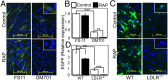
Vesicular stomatitis virus (VSV) exhibits a remarkably robust and pantropic infectivity, mediated by its coat protein, VSV-G. Using this property, recombinant forms of VSV and VSV-G-pseudotyped viral vectors are being developed for gene therapy, vaccination, and viral oncolysis and are extensively used for gene transduction in vivo and in vitro. The broad tropism of VSV suggests that it enters cells through a highly ubiquitous receptor, whose identity has so far remained elusive. Here we show that the LDL receptor (LDLR) serves as the major entry port of VSV and of VSV-G-pseudotyped lentiviral vectors in human and mouse cells, whereas other LDLR family members serve as alternative receptors. The widespread expression of LDLR family members accounts for the pantropism of VSV and for the broad applicability of VSV-G-pseudotyped viral vectors for gene transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: Re-inventing the bullet. Trends Mol Med. 2004;10(5):210–216. - PubMed
-
- Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. - PubMed
-
- Rose NF, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

