Phosphoinositides and membrane curvature switch the mode of actin polymerization via selective recruitment of toca-1 and Snx9
- PMID: 23589871
- PMCID: PMC3645562
- DOI: 10.1073/pnas.1305286110
Phosphoinositides and membrane curvature switch the mode of actin polymerization via selective recruitment of toca-1 and Snx9
Abstract
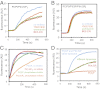
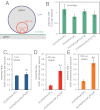
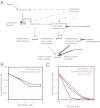
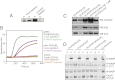
The membrane-cytosol interface is the major locus of control of actin polymerization. At this interface, phosphoinositides act as second messengers to recruit membrane-binding proteins. We show that curved membranes, but not flat ones, can use phosphatidylinositol 3-phosphate [PI(3)P] along with phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to stimulate actin polymerization. In this case, actin polymerization requires the small GTPase cell cycle division 42 (Cdc42), the nucleation-promoting factor neural Wiskott-Aldrich syndrome protein (N-WASP) and the actin nucleator the actin-related protein (Arp) 2/3 complex. In liposomes containing PI(4,5)P2 as the sole phosphoinositide, actin polymerization requires transducer of Cdc42 activation-1 (toca-1). In the presence of phosphatidylinositol 3-phosphate, polymerization is both more efficient and independent of toca-1. Under these conditions, sorting nexin 9 (Snx9) can be implicated as a specific adaptor that replaces toca-1 to mobilize neural Wiskott-Aldrich syndrome protein and the Arp2/3 complex. This switch in phosphoinositide and adaptor specificity for actin polymerization from membranes has implications for how different types of actin structures are generated at precise times and locations in the cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Triggering actin polymerization in Xenopus egg extracts from phosphoinositide-containing lipid bilayers.Methods Cell Biol. 2015;128:125-47. doi: 10.1016/bs.mcb.2015.01.020. Epub 2015 Apr 8. Methods Cell Biol. 2015. PMID: 25997346
-
Membrane composition and curvature in SNX9-mediated actin polymerization.Mol Biol Cell. 2025 May 1;36(5):ar54. doi: 10.1091/mbc.E24-09-0419. Epub 2025 Mar 19. Mol Biol Cell. 2025. PMID: 40105919
-
Control of actin polymerization via the coincidence of phosphoinositides and high membrane curvature.J Cell Biol. 2017 Nov 6;216(11):3745-3765. doi: 10.1083/jcb.201704061. Epub 2017 Sep 18. J Cell Biol. 2017. PMID: 28923975 Free PMC article.
-
Cdc42 in actin dynamics: An ordered pathway governed by complex equilibria and directional effector handover.Small GTPases. 2017 Oct 2;8(4):237-244. doi: 10.1080/21541248.2016.1215657. Epub 2016 Aug 11. Small GTPases. 2017. PMID: 27715449 Free PMC article. Review.
-
PIP3, PIP2, and cell movement--similar messages, different meanings?Dev Cell. 2001 Dec;1(6):743-7. doi: 10.1016/s1534-5807(01)00086-7. Dev Cell. 2001. PMID: 11740936 Free PMC article. Review.
Cited by
-
The phosphoinositide 3-kinase inhibitor alpelisib restores actin organization and improves proximal tubule dysfunction in vitro and in a mouse model of Lowe syndrome and Dent disease.Kidney Int. 2020 Oct;98(4):883-896. doi: 10.1016/j.kint.2020.05.040. Epub 2020 Sep 9. Kidney Int. 2020. PMID: 32919786 Free PMC article.
-
SNX3 drives maturation of Borrelia phagosomes by forming a hub for PI(3)P, Rab5a, and galectin-9.J Cell Biol. 2019 Sep 2;218(9):3039-3059. doi: 10.1083/jcb.201812106. Epub 2019 Jul 23. J Cell Biol. 2019. PMID: 31337623 Free PMC article.
-
Regulation of actin assembly by PI(4,5)P2 and other inositol phospholipids: An update on possible mechanisms.Biochem Biophys Res Commun. 2018 Nov 25;506(2):307-314. doi: 10.1016/j.bbrc.2018.07.155. Epub 2018 Aug 13. Biochem Biophys Res Commun. 2018. PMID: 30139519 Free PMC article. Review.
-
An autoinhibitory clamp of actin assembly constrains and directs synaptic endocytosis.Elife. 2021 Jul 29;10:e69597. doi: 10.7554/eLife.69597. Elife. 2021. PMID: 34324418 Free PMC article.
-
Investigation of the Interaction between Cdc42 and Its Effector TOCA1: HANDOVER OF Cdc42 TO THE ACTIN REGULATOR N-WASP IS FACILITATED BY DIFFERENTIAL BINDING AFFINITIES.J Biol Chem. 2016 Jun 24;291(26):13875-90. doi: 10.1074/jbc.M116.724294. Epub 2016 Apr 22. J Biol Chem. 2016. PMID: 27129201 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

