High-performance hollow sulfur nanostructured battery cathode through a scalable, room temperature, one-step, bottom-up approach
- PMID: 23589875
- PMCID: PMC3645569
- DOI: 10.1073/pnas.1220992110
High-performance hollow sulfur nanostructured battery cathode through a scalable, room temperature, one-step, bottom-up approach
Abstract
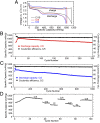
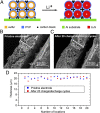
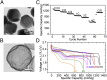
Sulfur is an exciting cathode material with high specific capacity of 1,673 mAh/g, more than five times the theoretical limits of its transition metal oxides counterpart. However, successful applications of sulfur cathode have been impeded by rapid capacity fading caused by multiple mechanisms, including large volume expansion during lithiation, dissolution of intermediate polysulfides, and low ionic/electronic conductivity. Tackling the sulfur cathode problems requires a multifaceted approach, which can simultaneously address the challenges mentioned above. Herein, we present a scalable, room temperature, one-step, bottom-up approach to fabricate monodisperse polymer (polyvinylpyrrolidone)-encapsulated hollow sulfur nanospheres for sulfur cathode, allowing unprecedented control over electrode design from nanoscale to macroscale. We demonstrate high specific discharge capacities at different current rates (1,179, 1,018, and 990 mAh/g at C/10, C/5, and C/2, respectively) and excellent capacity retention of 77.6% (at C/5) and 73.4% (at C/2) after 300 and 500 cycles, respectively. Over a long-term cycling of 1,000 cycles at C/2, a capacity decay as low as 0.046% per cycle and an average coulombic efficiency of 98.5% was achieved. In addition, a simple modification on the sulfur nanosphere surface with a layer of conducting polymer, poly(3,4-ethylenedioxythiophene), allows the sulfur cathode to achieve excellent high-rate capability, showing a high reversible capacity of 849 and 610 mAh/g at 2C and 4C, respectively.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Armand M, Tarascon JM. Building better batteries. Nature. 2008;451(7179):652–657. - PubMed
-
- Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: A battery of choices. Science. 2011;334(6058):928–935. - PubMed
-
- Whittingham MS. Lithium batteries and cathode materials. Chem Rev. 2004;104(10):4271–4301. - PubMed
-
- Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem Mater. 2010;22(3):587–603.
-
- Chan CK, et al. High-performance lithium battery anodes using silicon nanowires. Nat Nanotechnol. 2008;3(1):31–35. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

