Detergent-mediated incorporation of transmembrane proteins in giant unilamellar vesicles with controlled physiological contents
- PMID: 23589883
- PMCID: PMC3645586
- DOI: 10.1073/pnas.1303857110
Detergent-mediated incorporation of transmembrane proteins in giant unilamellar vesicles with controlled physiological contents
Abstract
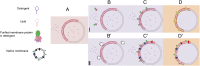
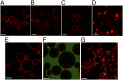
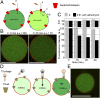
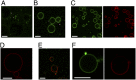
Giant unilamellar vesicles (GUVs) are convenient biomimetic systems of the same size as cells that are increasingly used to quantitatively address biophysical and biochemical processes related to cell functions. However, current approaches to incorporate transmembrane proteins in the membrane of GUVs are limited by the amphiphilic nature or proteins. Here, we report a method to incorporate transmembrane proteins in GUVs, based on concepts developed for detergent-mediated reconstitution in large unilamellar vesicles. Reconstitution is performed either by direct incorporation from proteins purified in detergent micelles or by fusion of purified native vesicles or proteoliposomes in preformed GUVs. Lipid compositions of the membrane and the ionic, protein, or DNA compositions in the internal and external volumes of GUVs can be controlled. Using confocal microscopy and functional assays, we show that proteins are unidirectionally incorporated in the GUVs and keep their functionality. We have successfully tested our method with three types of transmembrane proteins. GUVs containing bacteriorhodopsin, a photoactivable proton pump, can generate large transmembrane pH and potential gradients that are light-switchable and stable for hours. GUVs with FhuA, a bacterial porin, were used to follow the DNA injection by T5 phage upon binding to its transmembrane receptor. GUVs incorporating BmrC/BmrD, a bacterial heterodimeric ATP-binding cassette efflux transporter, were used to demonstrate the protein-dependent translocation of drugs and their interactions with encapsulated DNA. Our method should thus apply to a wide variety of membrane or peripheral proteins for producing more complex biomimetic GUVs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Schwille P. Bottom-up synthetic biology: Engineering in a tinkerer’s world. Science. 2011;333(6047):1252–1254. - PubMed
-
- Abkarian M, Loiseau E, Massiera G. Continuous droplet interface crossing encapsulation (cDICE) for high throughput monodisperse vesicle design. Soft Matter. 2011;7:4610–4614.
-
- Römer W, et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell. 2010;140(4):540–553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

