Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development
- PMID: 23589885
- PMCID: PMC3645587
- DOI: 10.1073/pnas.1220998110
Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development
Abstract
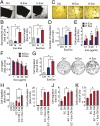
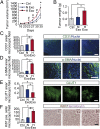
Hypoxia, or low oxygen tension, is a major regulator of tumor development and aggressiveness. However, how cancer cells adapt to hypoxia and communicate with their surrounding microenvironment during tumor development remain important questions. Here, we show that secreted vesicles with exosome characteristics mediate hypoxia-dependent intercellular signaling of the highly malignant brain tumor glioblastoma multiforme (GBM). In vitro hypoxia experiments with glioma cells and studies with patient materials reveal the enrichment in exosomes of hypoxia-regulated mRNAs and proteins (e.g., matrix metalloproteinases, IL-8, PDGFs, caveolin 1, and lysyl oxidase), several of which were associated with poor glioma patient prognosis. We show that exosomes derived from GBM cells grown at hypoxic compared with normoxic conditions are potent inducers of angiogenesis ex vivo and in vitro through phenotypic modulation of endothelial cells. Interestingly, endothelial cells were programmed by GBM cell-derived hypoxic exosomes to secrete several potent growth factors and cytokines and to stimulate pericyte PI3K/AKT signaling activation and migration. Moreover, exosomes derived from hypoxic compared with normoxic conditions showed increased autocrine, promigratory activation of GBM cells. These findings were correlated with significantly enhanced induction by hypoxic compared with normoxic exosomes of tumor vascularization, pericyte vessel coverage, GBM cell proliferation, as well as decreased tumor hypoxia in a mouse xenograft model. We conclude that the proteome and mRNA profiles of exosome vesicles closely reflect the oxygenation status of donor glioma cells and patient tumors, and that the exosomal pathway constitutes a potentially targetable driver of hypoxia-dependent intercellular signaling during tumor development.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2(1):38–47. - PubMed
-
- Pouysségur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441(7092):437–443. - PubMed
-
- Evans SM, et al. Hypoxia is important in the biology and aggression of human glial brain tumors. Clin Cancer Res. 2004;10(24):8177–8184. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

