Distinct glycosyltransferases synthesize E-selectin ligands in human vs. mouse leukocytes
- PMID: 23590904
- PMCID: PMC3711995
- DOI: 10.4161/cam.24714
Distinct glycosyltransferases synthesize E-selectin ligands in human vs. mouse leukocytes
Abstract
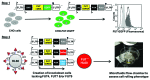
The binding of selectins to carbohydrate epitopes expressed on leukocytes is the first step in a multi-step cell adhesion cascade that controls the rate of leukocyte recruitment at sites of inflammation. The glycans that function as selectin-ligands are post-translationally synthesized by the serial action of Golgi resident enzymes called glycosyltransferases (glycoTs). Whereas much of our current knowledge regarding the role of glycoTs in constructing selectin-ligands comes from reconstituted biochemical investigations or murine models, tools to assess the impact of these enzymes on the human ligands are relatively underdeveloped. This is significant since the selectin-ligands, particularly those that bind E-selectin, vary between different leukocyte cell populations and they are also different in humans compared with mice. To address this shortcoming, a recent study by Buffone et al. (2013) outlines a systematic strategy to knockdown upto three glycoTs simultaneously in human leukocytes. The results suggest that the fucosyltransferases (FUTs) regulating selectin-ligand synthesis may be species-specific. In particular, they demonstrate that FUT9 plays a significant role during human, but not mouse, leukocyte-endothelial interactions. Overall, this article discusses the relative roles of the FUTs during human L-, E-, and P-selectin-ligand biosynthesis, and the potential that the knockdown strategy outlined here may assess the role of other glycoTs in human leukocytes also.
Keywords: carbohydrate; cell adhesion; endothelial cell; fluid shear; fucosyltransferase; glycosyltransferase; inflammation; leukocyte; selectin; sialyl Lewis-X.
Figures

Comment on
-
Silencing α1,3-fucosyltransferases in human leukocytes reveals a role for FUT9 enzyme during E-selectin-mediated cell adhesion.J Biol Chem. 2013 Jan 18;288(3):1620-33. doi: 10.1074/jbc.M112.400929. Epub 2012 Nov 28. J Biol Chem. 2013. PMID: 23192350 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
