Reaching to proprioceptively defined targets in Parkinson's disease: effects of deep brain stimulation therapy
- PMID: 23590906
- PMCID: PMC3780593
- DOI: 10.1016/j.neuroscience.2013.04.009
Reaching to proprioceptively defined targets in Parkinson's disease: effects of deep brain stimulation therapy
Abstract
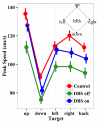
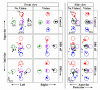
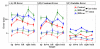
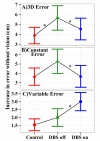
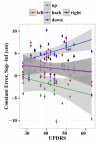
Deep brain stimulation of the subthalamic nucleus (STN DBS) provides a unique window into human brain function since it can reversibly alter the functioning of specific brain circuits. Basal ganglia-cortical circuits are thought to be excessively noisy in patients with Parkinson's disease (PD), based in part on the lack of specificity of proprioceptive signals in basal ganglia-thalamic-cortical circuits in monkey models of the disease. PD patients are known to have deficits in proprioception, but the effects are often subtle, with paradigms typically restricted to one or two joint movements in a plane. Moreover, the effects of STN DBS on proprioception are virtually unexplored. We tested the following hypotheses: first, that PD patients will show substantial deficits in unconstrained, multi-joint proprioception, and, second, that STN DBS will improve multi-joint proprioception. Twelve PD patients with bilaterally implanted electrodes in the subthalamic nucleus and 12 age-matched healthy subjects were asked to position the left hand at a location that was proprioceptively defined in 3D space with the right hand. In a second condition, subjects were provided visual feedback during the task so that they were not forced to rely on proprioception. Overall, with STN DBS switched off, PD patients showed significantly larger proprioceptive localization errors, and greater variability in endpoint localizations than the control subjects. Visual feedback partially normalized PD performance, and demonstrated that the errors in proprioceptive localization were not simply due to a difficulty in executing the movements or in remembering target locations. Switching STN DBS on significantly reduced localization errors from those of control subjects when patients moved without visual feedback relative to when they moved with visual feedback (when proprioception was not required). However, this reduction in localization errors without vision came at the cost of increased localization variability.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Abosch A, Hutchison WD, Saint-Cyr JA, Dostrovsky JO, Lozano AM. Movement-related neurons of the subthalamic nucleus in patients with Parkinson disease. J Neurosurg. 2002;97:1167–1172. - PubMed
-
- Adamovich SV, Berkinblit MB, Fookson O, Poizner H. Pointing in 3D space to remembered targets. I. Kinesthetic versus visual target presentation. J Neurophysiol. 1998;79:2833–2846. - PubMed
-
- Adamovich S, Berkinblit M, Hening W, Sage J, Poizner H. The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience. 2001;104:1027–1041. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

