Spinal mitochondrial-derived peroxynitrite enhances neuroimmune activation during morphine hyperalgesia and antinociceptive tolerance
- PMID: 23590939
- PMCID: PMC4874243
- DOI: 10.1016/j.pain.2013.02.018
Spinal mitochondrial-derived peroxynitrite enhances neuroimmune activation during morphine hyperalgesia and antinociceptive tolerance
Abstract
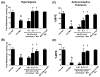
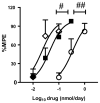
Treatment of severe pain by morphine, the gold-standard opioid and a potent drug in our arsenal of analgesic medications, is limited by the eventual development of hyperalgesia and analgesic tolerance. We recently reported that systemic administration of a peroxynitrite (PN) decomposition catalyst (PNDC) or superoxide dismutase mimetic attenuates morphine hyperalgesia and antinociceptive tolerance and reduces PN-mediated mitochondrial nitroxidative stress in the spinal cord. These results suggest the potential involvement of spinal PN signaling in this setting; which was examined in the present study. PN removal with intrathecal delivery of manganese porphyrin-based dual-activity superoxide/PNDCs, MnTE-2-PyP(5+) and the more lipophilic MnTnHex-2-PyP(5+), blocked hyperalgesia and antinociceptive tolerance in rats. Noteworthy is that intrathecal MnTnHex-2-PyP(5+) prevented nitration and inactivation of mitochondrial manganese superoxide dismutase. Mitochondrial manganese superoxide dismutase inactivation enhances the superoxide-to-PN pathway by preventing the dismutation of superoxide to hydrogen peroxide, thus providing an important enzymatic source for PN formation. Additionally, intrathecal MnTnHex-2-PyP(5+) attenuated neuroimmune activation by preventing the activation of nuclear factor kappa B, extracellular-signal-regulated kinase and p38 mitogen activated protein kinases, and the enhanced levels of proinflammatory cytokines, interleukin (IL)-1β and IL-6, while increasing anti-inflammatory cytokines, IL-4 and IL-10. The role of PN was further confirmed using intrathecal or oral delivery of the superoxide-sparing PNDC, SRI-110. These results suggest that mitochondrial-derived PN triggers the activation of several biochemical pathways engaged in the development of neuroinflammation in the spinal cord that are critical to morphine hyperalgesia and tolerance, further supporting the potential of targeting PN as an adjunct to opiates to maintain pain relief.
Copyright © 2013. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


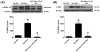
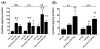


References
-
- Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Fridovich I. Manganese(III) meso tetrakis orthoN-alkylpyridylporphyrins. Synthesis, characterization and catalysis of O2 dismutation. J Chem Soc Dalton Trans. 2002;30:2689–96.
-
- Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7:161–9. - PubMed
-
- Chang G, Chen L, Mao J. Opioid tolerance and hyperalgesia. Med Clin North Am. 2007;91:199–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

