Identification of mitochondrial coenzyme a transporters from maize and Arabidopsis
- PMID: 23590975
- PMCID: PMC3668054
- DOI: 10.1104/pp.113.218081
Identification of mitochondrial coenzyme a transporters from maize and Arabidopsis
Abstract
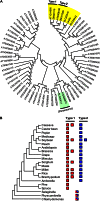
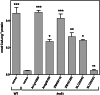
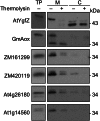
Plants make coenzyme A (CoA) in the cytoplasm but use it for reactions in mitochondria, chloroplasts, and peroxisomes, implying that these organelles have CoA transporters. A plant peroxisomal CoA transporter is already known, but plant mitochondrial or chloroplastic CoA transporters are not. Mitochondrial CoA transporters belonging to the mitochondrial carrier family, however, have been identified in yeast (Saccharomyces cerevisiae; Leu-5p) and mammals (SLC25A42). Comparative genomic analysis indicated that angiosperms have two distinct homologs of these mitochondrial CoA transporters, whereas nonflowering plants have only one. The homologs from maize (Zea mays; GRMZM2G161299 and GRMZM2G420119) and Arabidopsis (Arabidopsis thaliana; At1g14560 and At4g26180) all complemented the growth defect of the yeast leu5Δ mitochondrial CoA carrier mutant and substantially restored its mitochondrial CoA level, confirming that these proteins have CoA transport activity. Dual-import assays with purified pea (Pisum sativum) mitochondria and chloroplasts, and subcellular localization of green fluorescent protein fusions in transiently transformed tobacco (Nicotiana tabacum) Bright Yellow-2 cells, showed that the maize and Arabidopsis proteins are targeted to mitochondria. Consistent with the ubiquitous importance of CoA, the maize and Arabidopsis mitochondrial CoA transporter genes are expressed at similar levels throughout the plant. These data show that representatives of both monocotyledons and eudicotyledons have twin, mitochondrially located mitochondrial carrier family carriers for CoA. The highly conserved nature of these carriers makes possible their reliable annotation in other angiosperm genomes.
Figures


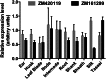
References
-
- Agrimi G, Russo A, Pierri CL, Palmieri F. (2012a) The peroxisomal NAD+ carrier of Arabidopsis thaliana transports coenzyme A and its derivatives. J Bioenerg Biomembr 44: 333–340 - PubMed
-
- Agrimi G, Russo A, Scarcia P, Palmieri F. (2012b) The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+. Biochem J 443: 241–247 - PubMed
-
- Bedhomme M, Hoffmann M, McCarthy EA, Gambonnet B, Moran RG, Rébeillé F, Ravanel S. (2005) Folate metabolism in plants: an Arabidopsis homolog of the mammalian mitochondrial folate transporter mediates folate import into chloroplasts. J Biol Chem 280: 34823–34831 - PubMed
-
- Bernhardt K, Wilkinson S, Weber AP, Linka N. (2012) A peroxisomal carrier delivers NAD+ and contributes to optimal fatty acid degradation during storage oil mobilization. Plant J 69: 1–13 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

