Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success
- PMID: 23591016
- PMCID: PMC3679282
- DOI: 10.1016/j.yjmcc.2013.04.004
Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success
Abstract
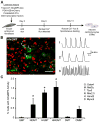
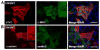
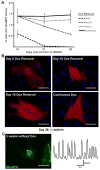
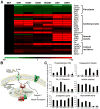
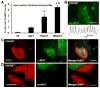
Direct conversion of fibroblasts to induced cardiomyocytes (iCMs) has great potential for regenerative medicine. Recent publications have reported significant progress, but the evaluation of reprogramming has relied upon non-functional measures such as flow cytometry for cardiomyocyte markers or GFP expression driven by a cardiomyocyte-specific promoter. The issue is one of practicality: the most stringent measures - electrophysiology to detect cell excitation and the presence of spontaneously contracting myocytes - are not readily quantifiable in the large numbers of cells screened in reprogramming experiments. However, excitation and contraction are linked by a third functional characteristic of cardiomyocytes: the rhythmic oscillation of intracellular calcium levels. We set out to optimize direct conversion of fibroblasts to iCMs with a quantifiable calcium reporter to rapidly assess functional transdifferentiation. We constructed a reporter system in which the calcium indicator GCaMP is driven by the cardiomyocyte-specific Troponin T promoter. Using calcium activity as our primary outcome measure, we compared several published combinations of transcription factors along with novel combinations in mouse embryonic fibroblasts. The most effective combination consisted of Hand2, Nkx2.5, Gata4, Mef2c, and Tbx5 (HNGMT). This combination is >50-fold more efficient than GMT alone and produces iCMs with cardiomyocyte marker expression, robust calcium oscillation, and spontaneous beating that persist for weeks following inactivation of reprogramming factors. HNGMT is also significantly more effective than previously published factor combinations for the transdifferentiation of adult mouse cardiac fibroblasts to iCMs. Quantification of calcium function is a convenient and effective means for the identification and evaluation of cardiomyocytes generated by direct reprogramming. Using this stringent outcome measure, we conclude that HNGMT produces iCMs more efficiently than previously published methods.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Protze S, Khattak S, Poulet C, Lindemann D, Tanaka EM, Ravens U. A new approach to transcription factor screening for reprogramming of fibroblasts to cardiomyocyte-like cells. J Mol Cell Cardiol. 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

