Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors
- PMID: 23591719
- PMCID: PMC3628113
- DOI: 10.1038/srep01673
Thrombospondin-1 signaling through CD47 inhibits self-renewal by regulating c-Myc and other stem cell transcription factors
Abstract
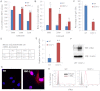
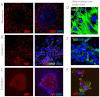
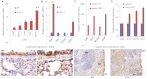
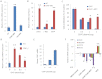
Signaling through the thrombospondin-1 receptor CD47 broadly limits cell and tissue survival of stress, but the molecular mechanisms are incompletely understood. We now show that loss of CD47 permits sustained proliferation of primary murine endothelial cells, increases asymmetric division, and enables these cells to spontaneously reprogram to form multipotent embryoid body-like clusters. c-Myc, Klf4, Oct4, and Sox2 expression is elevated in CD47-null endothelial cells, in several tissues of CD47- and thrombospondin-1-null mice, and in a human T cell line lacking CD47. CD47 knockdown acutely increases mRNA levels of c-Myc and other stem cell transcription factors in cells and in vivo, whereas CD47 ligation by thrombospondin-1 suppresses c-Myc expression. The inhibitory effects of increasing CD47 levels can be overcome by maintaining c-Myc expression and are absent in cells with dysregulated c-Myc. Thus, CD47 antagonists enable cell self-renewal and reprogramming by overcoming negative regulation of c-Myc and other stem cell transcription factors.
Figures


References
-
- Matozaki T., Murata Y., Okazawa H. & Ohnishi H. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol 19, 72–80 (2009). - PubMed
-
- Frazier W. A., Isenberg J. S., Kaur S. & Roberts D. D. in UCSD Nature Molecule Pages (2010). doi:10.1038/mp.a002870.01.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

