Association between Gαi2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis
- PMID: 23591873
- PMCID: PMC3644068
- DOI: 10.1038/ncomms2680
Association between Gαi2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis
Abstract
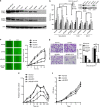
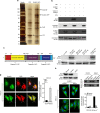
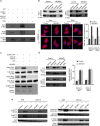
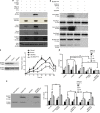
The chemokine CXCL12 and its G-protein-coupled receptor CXCR4 control the migration, invasiveness and metastasis of breast cancer cells. Binding of CXCL12 to CXCR4 triggers activation of heterotrimeric Gi proteins that regulate actin polymerization and migration. However, the pathways linking chemokine G-protein-coupled receptor/Gi signalling to actin polymerization and cancer cell migration are not known. Here we show that CXCL12 stimulation promotes interaction between Gαi2 and ELMO1. Gi signalling and ELMO1 are both required for CXCL12-mediated actin polymerization, migration and invasion of breast cancer cells. CXCL12 triggers a Gαi2-dependent membrane translocation of ELMO1, which associates with Dock180 to activate small G-proteins Rac1 and Rac2. In vivo, ELMO1 expression is associated with lymph node and distant metastasis, and knocking down ELMO1 impairs metastasis to the lung. Our findings indicate that a chemokine-controlled pathway, consisting of Gαi2, ELMO1/Dock180, Rac1 and Rac2, regulates the actin cytoskeleton during breast cancer metastasis.
Figures

References
-
- Muller A. et al.. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001). - PubMed
-
- Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib. Microbiol. 13, 191–199 (2006). - PubMed
-
- Nicolson G. L. Paracrine and autocrine growth mechanisms in tumor metastasis to specific sites with particular emphasis on brain and lung metastasis. Cancer Metastasis Rev. 12, 325–343 (1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

