Arginine clustering on calix[4]arene macrocycles for improved cell penetration and DNA delivery
- PMID: 23591888
- PMCID: PMC3644092
- DOI: 10.1038/ncomms2721
Arginine clustering on calix[4]arene macrocycles for improved cell penetration and DNA delivery
Abstract
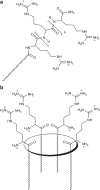
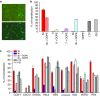
Cell-penetrating peptides are widely used as molecular transporters for the internalization inside cells of various cargo, including proteins and nucleic acids. A special role is played by arginine-rich peptides and oligoarginines covalently linked or simply mixed with the cargo. Here we report cell-penetrating agents in which arginine units are clustered on a macrocyclic scaffold. Instead of using long peptides, four single arginine units were covalently attached to either the upper or lower rim of a calix[4]arene, kept in the cone conformation building a 'parallel' cyclic array. These new macrocyclic carriers show high efficiency in DNA delivery and transfection in a variety of cell lines.
Figures

Similar articles
-
Gene delivery based on macrocyclic amphiphiles.Theranostics. 2019 May 18;9(11):3094-3106. doi: 10.7150/thno.31914. eCollection 2019. Theranostics. 2019. PMID: 31244943 Free PMC article. Review.
-
Macrocyclic nonviral vectors: high cell transfection efficiency and low toxicity in a lower rim guanidinium calix[4]arene.Org Lett. 2008 Sep 18;10(18):3953-6. doi: 10.1021/ol801326d. Epub 2008 Aug 23. Org Lett. 2008. PMID: 18722463
-
Design and synthesis of biologically active cationic amphiphiles built on the calix[4]arene scaffold.Int J Pharm. 2018 Oct 5;549(1-2):436-445. doi: 10.1016/j.ijpharm.2018.08.020. Epub 2018 Aug 14. Int J Pharm. 2018. PMID: 30118833
-
Lower rim guanidinocalix[4]arenes: macrocyclic nonviral vectors for cell transfection.Bioconjug Chem. 2012 May 16;23(5):993-1002. doi: 10.1021/bc2006829. Epub 2012 Apr 16. Bioconjug Chem. 2012. PMID: 22463059
-
Biomimetic and self-assembled calix[6]arene-based receptors for neutral molecules.Org Biomol Chem. 2009 Jun 21;7(12):2485-500. doi: 10.1039/b902456e. Epub 2009 Apr 23. Org Biomol Chem. 2009. PMID: 19503918 Review.
Cited by
-
Gene delivery based on macrocyclic amphiphiles.Theranostics. 2019 May 18;9(11):3094-3106. doi: 10.7150/thno.31914. eCollection 2019. Theranostics. 2019. PMID: 31244943 Free PMC article. Review.
-
Cell-Penetrating Peptides.Methods Mol Biol. 2022;2383:3-32. doi: 10.1007/978-1-0716-1752-6_1. Methods Mol Biol. 2022. PMID: 34766279
-
Formation of functional super-helical assemblies by constrained single heptad repeat.Nat Commun. 2015 Oct 15;6:8615. doi: 10.1038/ncomms9615. Nat Commun. 2015. PMID: 26468599 Free PMC article.
-
Efficient Delivery of MicroRNA and AntimiRNA Molecules Using an Argininocalix[4]arene Macrocycle.Mol Ther Nucleic Acids. 2019 Dec 6;18:748-763. doi: 10.1016/j.omtn.2019.09.029. Epub 2019 Oct 11. Mol Ther Nucleic Acids. 2019. PMID: 31733592 Free PMC article.
-
On guanidinium and cellular uptake.J Org Chem. 2014 Aug 1;79(15):6766-74. doi: 10.1021/jo501101s. Epub 2014 Jul 21. J Org Chem. 2014. PMID: 25019333 Free PMC article.
References
-
- Futaki S. et al.. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 276, 5836–5840 (2001) . - PubMed
-
- Nakase I., Takeuchi T., Tanaka G. & Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv. Drug Deliv. Rev. 60, 598–607 (2008) . - PubMed
-
- Walrant A., Bechara C., Alves I. D. & Sagan S. Molecular partners for interaction and cell internalization of cell-penetrating peptides: how identical are they? Nanomedicine 7, 133–143 (2012) . - PubMed
-
- Vivès E., Brodin P. & Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272, 16010–16017 (1997) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

