Molecular mechanics of mineralized collagen fibrils in bone
- PMID: 23591891
- PMCID: PMC3644085
- DOI: 10.1038/ncomms2720
Molecular mechanics of mineralized collagen fibrils in bone
Abstract
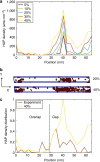
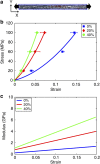
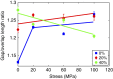
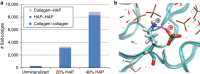
Bone is a natural composite of collagen protein and the mineral hydroxyapatite. The structure of bone is known to be important to its load-bearing characteristics, but relatively little is known about this structure or the mechanism that govern deformation at the molecular scale. Here we perform full-atomistic calculations of the three-dimensional molecular structure of a mineralized collagen protein matrix to try to better understand its mechanical characteristics under tensile loading at various mineral densities. We find that as the mineral density increases, the tensile modulus of the network increases monotonically and well beyond that of pure collagen fibrils. Our results suggest that the mineral crystals within this network bears up to four times the stress of the collagen fibrils, whereas the collagen is predominantly responsible for the material's deformation response. These findings reveal the mechanism by which bone is able to achieve superior energy dissipation and fracture resistance characteristics beyond its individual constituents.
Figures


References
-
- Fratzl P. (ed.). Collagen: Structure and Mechanics (Springer (2008)).
-
- Fratzl P., Gupta H. S., Paschalis E. P. & Roschger P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J. Mater. Chem. 14, 2115–2123 (2004).
-
- Fratzl P. & Weinkamer R. Nature’s hierarchical materials. Prog. Mater. Sci. 52, 1263–1334 (2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

