A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils
- PMID: 23592514
- PMCID: PMC3684828
- DOI: 10.1124/mol.113.085654
A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils
Abstract
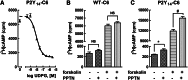
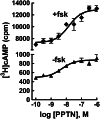
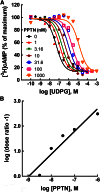
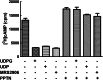
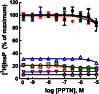
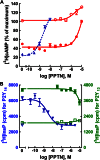
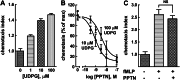
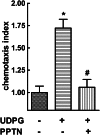
The nucleotide-sugar-activated P2Y14 receptor (P2Y14-R) is highly expressed in hematopoietic cells. Although the physiologic functions of this receptor remain undefined, it has been strongly implicated recently in immune and inflammatory responses. Lack of availability of receptor-selective high-affinity antagonists has impeded progress in studies of this and most of the eight nucleotide-activated P2Y receptors. A series of molecules recently were identified by Gauthier et al. (Gauthier et al., 2011) that exhibited antagonist activity at the P2Y14-R. We synthesized one of these molecules, a 4,7-disubstituted 2-naphthoic acid derivative (PPTN), and studied its pharmacological properties in detail. The concentration-effect curve of UDP-glucose for promoting inhibition of adenylyl cyclase in C6 glioma cells stably expressing the P2Y14-R was shifted to the right in a concentration-dependent manner by PPTN. Schild analyses revealed that PPTN-mediated inhibition followed competitive kinetics, with a KB of 434 pM observed. In contrast, 1 μM PPTN exhibited no agonist or antagonist effect at the P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, or P2Y13 receptors. UDP-glucose-promoted chemotaxis of differentiated HL-60 human promyelocytic leukemia cells was blocked by PPTN with a concentration dependence consistent with the KB determined with recombinant P2Y14-R. In contrast, the chemotactic response evoked by the chemoattractant peptide fMetLeuPhe was unaffected by PPTN. UDP-glucose-promoted chemotaxis of freshly isolated human neutrophils also was blocked by PPTN. In summary, this work establishes PPTN as a highly selective high-affinity antagonist of the P2Y14-R that is useful for interrogating the action of this receptor in physiologic systems.
Figures
References
-
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, et al. (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341 - PMC - PubMed
-
- Arase T, Uchida H, Kajitani T, Ono M, Tamaki K, Oda H, Nishikawa S, Kagami M, Nagashima T, Masuda H, et al. (2009) The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J Immunol 182:7074–7084 - PubMed
-
- Belly M, Deschenes D, Fortin R, Fournier JF, Gagne S, Gareau Y, Gautheir JY, Li L, Robichaud J, Therien M, Tranmer GK, and Wang Z (2009) inventors, Merck Frosst Canada LTD, assignee. Substituted 2-naphthoic acids as antagonists of GPR105 activity. WO 2009/070873A1. 2010 Nov 25.
-
- Boger DL, Han N, Tarby CM, Boyce CW, Cai H, Jin Q, Kitos PA. (1996) Synthesis, chemical properties, and preliminary evaluation of substituted CBI analogs of CC-1065 and the duocarmycins incorporating the 7-cyano-1,2,9,9a-tetrahydrocyclopropa[c]benz[e]indol-4-one alkylation subunit: Hammett quantitation of the magnitude of electronic effects on functional reactivity. J Org Chem 61:4894–4912 - PubMed
-
- Brown HA, Lazarowski ER, Boucher RC, Harden TK. (1991) Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5′-nucleotide receptor in human airway epithelial cells. Mol Pharmacol 40:648–655 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

