Facilitated hyperpolarization signaling in vascular smooth muscle-overexpressing TRIC-A channels
- PMID: 23592776
- PMCID: PMC3668719
- DOI: 10.1074/jbc.M112.435396
Facilitated hyperpolarization signaling in vascular smooth muscle-overexpressing TRIC-A channels
Abstract
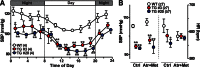
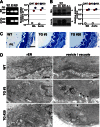
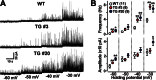
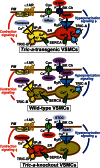
The TRIC channel subtypes, namely TRIC-A and TRIC-B, are intracellular monovalent cation-specific channels and likely mediate counterion movements to support efficient Ca(2+) release from the sarco/endoplasmic reticulum. Vascular smooth muscle cells (VSMCs) contain both TRIC subtypes and two Ca(2+) release mechanisms; incidental opening of ryanodine receptors (RyRs) generates local Ca(2+) sparks to induce hyperpolarization and relaxation, whereas agonist-induced activation of inositol trisphosphate receptors produces global Ca(2+) transients causing contraction. Tric-a knock-out mice develop hypertension due to insufficient RyR-mediated Ca(2+) sparks in VSMCs. Here we describe transgenic mice overexpressing TRIC-A channels under the control of a smooth muscle cell-specific promoter. The transgenic mice developed congenital hypotension. In Tric-a-overexpressing VSMCs from the transgenic mice, the resting membrane potential decreased because RyR-mediated Ca(2+) sparks were facilitated and cell surface Ca(2+)-dependent K(+) channels were hyperactivated. Under such hyperpolarized conditions, L-type Ca(2+) channels were inactivated, and thus, the resting intracellular Ca(2+) levels were reduced in Tric-a-overexpressing VSMCs. Moreover, Tric-a overexpression impaired inositol trisphosphate-sensitive stores to diminish agonist-induced Ca(2+) signaling in VSMCs. These altered features likely reduced vascular tonus leading to the hypotensive phenotype. Our Tric-a-transgenic mice together with Tric-a knock-out mice indicate that TRIC-A channel density in VSMCs is responsible for controlling basal blood pressure at the whole-animal level.
Keywords: Blood Pressure; Ca2+ Spark; Ca2+-dependent K+ Channel; Calcium Imaging; Calcium Intracellular Release; Inositol 1,4,5-Trisphosphate receptor; Potassium Channels; Ryanodine Receptor; TRIC Channel; Vascular Smooth Muscle Cells.
Figures



References
-
- Bezprozvanny I., Ehrlich B. E. (1995) The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 145, 205–216 - PubMed
-
- Meissner G. (1994) Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 56, 485–508 - PubMed
-
- Wellman G. C., Nelson M. T. (2003) Signaling between SR and plasmalemma in smooth muscle. Sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium 34, 211–229 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

