Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort
- PMID: 23592832
- PMCID: PMC5672918
- DOI: 10.1093/cid/cit245
Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort
Abstract
Background: Liver biopsy remains critical for staging liver disease in hepatitis C virus (HCV)-infected persons, but is a bottleneck to evaluation, follow-up, and treatment of HCV. Our analysis sought to validate APRI (aspartate aminotransferase [AST]-to-platelet ratio index) and FIB-4, an index from serum fibrosis markers (alanine aminotransferase [ALT], AST, and platelets plus patient age) to stage liver disease.
Methods: Biopsy results from HCV patients in the Chronic Hepatitis Cohort Study were mapped to an F0-F4 equivalent scale; APRI and FIB-4 scores at the time of biopsy were then mapped to the same scale.
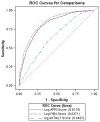
Results: We identified 2372 liver biopsies from HCV-infected patients with contemporaneous laboratory values for imputing APRI and FIB-4. Fibrosis stage distributions by the equivalent biopsy scale were 267 (11%) F0; 555 (23%) F1; 648 (27%) F2; 394 (17%) F3; and 508 (21%) F4. Mean APRI and FIB-4 values significantly increased with successive fibrosis levels (P < .05). The areas under the receiver operating characteristic curve (AUROC) analysis distinguishing severe (F3-F4) from mild-to-moderate fibrosis (F0-F2) were 0.80 (95% confidence interval [CI], .78-.82) for APRI and 0.83 (95% CI, .81-.85) for FIB-4. There was a significant difference between the AUROCs of FIB-4 and APRI (P < .001); 88% of persons who had a FIB-4 score ≥2.0 were at stage F2 or higher.
Conclusions: In a large observational cohort, FIB-4 was good at differentiating 5 stages of chronic HCV infection. It can be useful in screening patients who need biopsy and therapy, for monitoring patients with less advanced disease, and for longitudinal studies.
Keywords: chronic hepatitis; clinical staging; hepatitis C virus.
Conflict of interest statement
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicenter retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–73. - PubMed
-
- Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–8. - PubMed
-
- Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–57. - PubMed
-
- Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterol. 2012;142:1293–302. - PubMed
-
- Wai C-T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatol. 2003;38:518–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

