Profiling of genes associated with the murine model of oxygen-induced retinopathy
- PMID: 23592914
- PMCID: PMC3626293
Profiling of genes associated with the murine model of oxygen-induced retinopathy
Abstract
Purpose: To compare the clinical features and gene expression patterns of the physiologic development of retinal vessels and oxygen-induced retinopathy (OIR) in a mouse model, with the aim of identifying differential regulators of physiologic and pathological angiogenesis in the retina.
Methods: C57BL/6J mice were used. Seven-day-old pups were subjected to OIR induction following the standard protocols of entering a hyperoxic chamber on day 7 (P7) and returning to a normoxic condition (relative hypoxia) on day 12 (P12). The retinal vasculatures in the OIR model 24 h (P8-O) or 5 days (P12-O) after switching to the hyperoxic environment and 24 h (P13-O) after returning to normoxic conditions were evaluated with retinal flat mounts and compared with those of age-matched controls (i.e., P8-N, P12-N, P13-N). Gene expression profiling was performed using Phalanx Mouse Whole Genome OneArray microarrays. Normal 9-day-old mice were considered representative of physiologic angiogenesis and compared with 30-day-old mice. A bioinformatics analysis was performed on differentially expressed genes using various comparisons, and real-time reverse-transcription PCR was used to confirm the changes in the genes of interest.
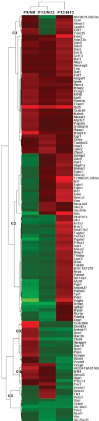
Results: The sequential orders and patterns of vasculature development in normal mice and the OIR models were significantly different. In brief, in the early days (P1 to P7) for normal mice, retinal vessels grew from the optic disc into the non-vascularized retina in a radial fashion. In the hyperoxic stage of the OIR model, the main central retina became devoid of a vascular network, and when the mice returned to the normoxic room, the vessels grew from peripheral perfused areas toward the center of the retina, but the development of intermediate and deep layers of vasculature was significantly delayed. Gene profiling at three critical time points (P8, P12, and P13) showed that 162 probes were upregulated to ≥1.5-fold or downregulated to ≤0.67-fold at one or more time points in the OIR model compared to the controls. In the 45 upregulated genes for the P8-O/P8-N group, enriched genes were mainly related to cytoskeleton formation, whereas the 62 upregulated genes for P13-O/P13-N participated in various pathological processes. In the physiologic conditions on P9, however, 135 genes were upregulated compared with P30; the gap junction and Fc gamma R-mediated phagocytosis were the two main enriched pathways for these genes. Fifty-three probes, including vascular endothelium growth factor A, annexin A2, and endothelin 2, changed at P13-O but not at P9-N, and these changed genes might reflect the modulation of pathological neovascularization.
Conclusions: Angiogenesis in physiologic and pathological conditions is characterized by the differential presentation of vasculature and gene expression patterns. Investigation of those genes unique to the OIR model may help develop new strategies and therapies for intervening in retinal neovascularization.
Figures




Similar articles
-
Comprehensive gene-expression profile in murine oxygen-induced retinopathy.Br J Ophthalmol. 2009 Jan;93(1):96-103. doi: 10.1136/bjo.2008.142646. Epub 2008 Oct 6. Br J Ophthalmol. 2009. PMID: 18838407
-
Gene expression profile of hyperoxic and hypoxic retinas in a mouse model of oxygen-induced retinopathy.Invest Ophthalmol Vis Sci. 2010 Aug;51(8):4307-19. doi: 10.1167/iovs.09-4605. Epub 2010 Mar 10. Invest Ophthalmol Vis Sci. 2010. PMID: 20220049
-
Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model.Graefes Arch Clin Exp Ophthalmol. 2009 Sep;247(9):1205-11. doi: 10.1007/s00417-009-1116-4. Epub 2009 Jun 13. Graefes Arch Clin Exp Ophthalmol. 2009. PMID: 19526246
-
Role of the retinal vascular endothelial cell in ocular disease.Prog Retin Eye Res. 2013 Jan;32:102-80. doi: 10.1016/j.preteyeres.2012.08.004. Epub 2012 Sep 11. Prog Retin Eye Res. 2013. PMID: 22982179 Free PMC article. Review.
-
Targeting Neurovascular Interaction in Retinal Disorders.Int J Mol Sci. 2020 Feb 22;21(4):1503. doi: 10.3390/ijms21041503. Int J Mol Sci. 2020. PMID: 32098361 Free PMC article. Review.
Cited by
-
Identification of immune associated potential molecular targets in proliferative diabetic retinopathy.BMC Ophthalmol. 2023 Jan 19;23(1):27. doi: 10.1186/s12886-023-02774-y. BMC Ophthalmol. 2023. PMID: 36658547 Free PMC article.
-
Transcriptome analysis of AAV-induced retinopathy models expressing human VEGF, TNF-α, and IL-6 in murine eyes.Sci Rep. 2022 Nov 12;12(1):19395. doi: 10.1038/s41598-022-23065-4. Sci Rep. 2022. PMID: 36371417 Free PMC article.
-
Short- and long-term impact of hyperoxia on the blood and retinal cells' transcriptome in a mouse model of oxygen-induced retinopathy.Pediatr Res. 2020 Feb;87(3):485-493. doi: 10.1038/s41390-019-0598-y. Epub 2019 Oct 2. Pediatr Res. 2020. PMID: 31578039 Free PMC article.
-
Diabetes Induced Alterations in Murine Vitreous Proteome Are Mitigated by IL-6 Trans-Signaling Inhibition.Invest Ophthalmol Vis Sci. 2020 Sep 1;61(11):2. doi: 10.1167/iovs.61.11.2. Invest Ophthalmol Vis Sci. 2020. PMID: 32870245 Free PMC article.
-
The Proteome of Native Adult Müller Glial Cells From Murine Retina.Mol Cell Proteomics. 2016 Feb;15(2):462-80. doi: 10.1074/mcp.M115.052183. Epub 2015 Aug 31. Mol Cell Proteomics. 2016. PMID: 26324419 Free PMC article.
References
-
- Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y. Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:393–402. - PubMed
-
- Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, Hackett SF, Okamoto N, Zack DJ, Semenza GL, Campochiaro PA. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–9. - PubMed
-
- Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H. Suppression of retinal neovascularization by the NF-kappaB inhibitor pyrrolidine dithiocarbamate in mice. Invest Ophthalmol Vis Sci. 1999;40:1624–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
