Drosophila DJ-1 decreases neural sensitivity to stress by negatively regulating Daxx-like protein through dFOXO
- PMID: 23593018
- PMCID: PMC3616925
- DOI: 10.1371/journal.pgen.1003412
Drosophila DJ-1 decreases neural sensitivity to stress by negatively regulating Daxx-like protein through dFOXO
Abstract
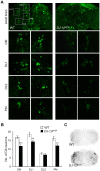
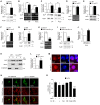
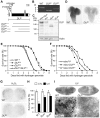
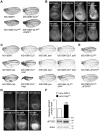
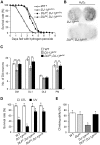
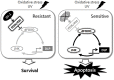
DJ-1, a Parkinson's disease (PD)-associated gene, has been shown to protect against oxidative stress in Drosophila. However, the molecular mechanism underlying oxidative stress-induced phenotypes, including apoptosis, locomotive defects, and lethality, in DJ-1-deficient flies is not fully understood. Here we showed that Daxx-like protein (DLP), a Drosophila homologue of the mammalian Death domain-associated protein (Daxx), was upregulated under oxidative stress conditions in the loss-of-function mutants of Drosophila DJ-1β, a Drosophila homologue of DJ-1. DLP overexpression induced apoptosis via the c-Jun N-terminal kinase (JNK)/Drosophila forkhead box subgroup O (dFOXO) pathway, whereas loss of DLP increased resistance to oxidative stress and UV irradiation. Moreover, the oxidative stress-induced phenotypes of DJ-1β mutants were dramatically rescued by DLP deficiency, suggesting that enhanced expression of DLP contributes to the DJ-1β mutant phenotypes. Interestingly, we found that dFOXO was required for the increase in DLP expression in DJ-1β mutants and that dFOXO activity was increased in the heads of DJ-1β mutants. In addition, subcellular localization of DLP appeared to be influenced by DJ-1 expression so that cytosolic DLP was increased in DJ-1β mutants. Similarly, in mammalian cells, Daxx translocation from the nucleus to the cytosol was suppressed by overexpressed DJ-1β under oxidative stress conditions; and, furthermore, targeted expression of DJ-1β to mitochondria efficiently inhibited the Daxx translocation. Taken together, our findings demonstrate that DJ-1β protects flies against oxidative stress- and UV-induced apoptosis by regulating the subcellular localization and gene expression of DLP, thus implying that Daxx-induced apoptosis is involved in the pathogenesis of DJ-1-associated PD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Daxx-like protein of Drosophila interacts with Dmp53 and affects longevity and Ark mRNA level.J Biol Chem. 2007 Dec 14;282(50):36386-93. doi: 10.1074/jbc.M705547200. Epub 2007 Oct 12. J Biol Chem. 2007. PMID: 17933869
-
Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction.Gene. 2005 Nov 21;361:133-9. doi: 10.1016/j.gene.2005.06.040. Epub 2005 Oct 3. Gene. 2005. PMID: 16203113
-
Effects of pharmacological agents on the lifespan phenotype of Drosophila DJ-1beta mutants.Gene. 2010 Aug 15;462(1-2):26-33. doi: 10.1016/j.gene.2010.04.009. Epub 2010 Apr 24. Gene. 2010. PMID: 20423725
-
Lessons from Drosophila models of DJ-1 deficiency.Sci Aging Knowledge Environ. 2006 Jan 11;2006(2):pe2. doi: 10.1126/sageke.2006.2.pe2. Sci Aging Knowledge Environ. 2006. PMID: 16407572 Review.
-
Role of DJ-1 in the mechanism of pathogenesis of Parkinson's disease.J Bioenerg Biomembr. 2019 Jun;51(3):175-188. doi: 10.1007/s10863-019-09798-4. Epub 2019 May 3. J Bioenerg Biomembr. 2019. PMID: 31054074 Free PMC article. Review.
Cited by
-
Phenotypic differences between Drosophila Alzheimer's disease models expressing human Aβ42 in the developing eye and brain.Anim Cells Syst (Seoul). 2017 Apr 15;21(3):160-168. doi: 10.1080/19768354.2017.1313777. eCollection 2017. Anim Cells Syst (Seoul). 2017. PMID: 30460065 Free PMC article.
-
Pyruvate Dehydrogenase Kinase Protects Dopaminergic Neurons from Oxidative Stress in Drosophila DJ-1 Null Mutants.Mol Cells. 2022 Jul 31;45(7):454-464. doi: 10.14348/molcells.2022.5002. Epub 2022 Apr 21. Mol Cells. 2022. PMID: 35444068 Free PMC article.
-
The Dynamic Regulation of Daxx-Mediated Transcriptional Inhibition by SUMO and PML NBs.Int J Mol Sci. 2025 Jul 12;26(14):6703. doi: 10.3390/ijms26146703. Int J Mol Sci. 2025. PMID: 40724953 Free PMC article. Review.
-
The Parkinson's disease gene product DJ-1 modulates miR-221 to promote neuronal survival against oxidative stress.Redox Biol. 2018 Oct;19:62-73. doi: 10.1016/j.redox.2018.07.021. Epub 2018 Aug 3. Redox Biol. 2018. PMID: 30107296 Free PMC article.
-
Impaired Taste Associative Memory and Memory Enhancement by Feeding Omija in Parkinson's Disease Fly Model.Mol Cells. 2018 Jul 31;41(7):646-652. doi: 10.14348/molcells.2018.0014. Epub 2018 Jun 25. Mol Cells. 2018. PMID: 29936793 Free PMC article.
References
-
- Emerit J, Edeas M, Bricaire F (2004) Neurodegenerative diseases and oxidative stress. Biomed Pharmacother 58: 39–46. - PubMed
-
- Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787–795. - PubMed
-
- Tsang AHK, Chung KKK (2009) Oxidative and nitrosative stress in Parkinson's disease. Biochim Biophys Acta 1792: 643–650. - PubMed
-
- Jenner P (2007) Oxidative stress and Parkinson's disease. Handb Clin Neurol 83: 507–520. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

