Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study
- PMID: 23593142
- PMCID: PMC3622027
- DOI: 10.1371/journal.pone.0059524
Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study
Abstract
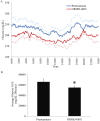
The unpredictable behavior of uncontrolled type 1 diabetes often involves frequent swings in blood glucose levels that impact maintenance of a daily routine. An intensified insulin regimen is often unsuccessful, while other therapeutic options, such as amylin analog injections, use of continuous glucose sensors, and islet or pancreas transplantation are of limited clinical use. In efforts to provide patients with a more compliable treatment method, Oramed Pharmaceuticals tested the capacity of its oral insulin capsule (ORMD-0801, 8 mg insulin) in addressing this resistant clinical state. Eight Type I diabetes patients with uncontrolled diabetes (HbA1c: 7.5-10%) were monitored throughout the 15-day study period by means of a blind continuous glucose monitoring device. Baseline patient blood glucose behavior was monitored and recorded over a five-day pretreatment screening period. During the ensuing ten-day treatment phase, patients were asked to conduct themselves as usual and to self-administer an oral insulin capsule three times daily, just prior to meal intake. CGM data sufficient for pharmacodynamics analyses were obtained from 6 of the 8 subjects. Treatment with ORMD-0801 was associated with a significant 24.4% reduction in the frequencies of glucose readings >200 mg/dL (60.1 ± 7.9% pretreatment vs. 45.4 ± 4.9% during ORMD-0801 treatment; p = 0.023) and a significant mean 16.6% decrease in glucose area under the curve (AUC) (66055 ± 5547 mg/dL/24 hours vs. 55060 ± 3068 mg/dL/24 hours, p = 0.023), with a greater decrease during the early evening hours. In conclusion, ORMD-0801 oral insulin capsules in conjunction with subcutaneous insulin injections, well tolerated and effectively reduced glycemia throughout the day.
Trial registration: Clinicaltrials.gov NCT00867594.
Conflict of interest statement
Figures
References
-
- Schade DS, Burge MR (1995) Brittle diabetes: etiology and treatment. Adv Endocrinol Metab 6: 289–319. - PubMed
-
- DeFronzo RA, Simonson D, Ferrannini E (1982) Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia 23: 313–319. - PubMed
-
- Meyer C, Dostou JM, Welle SL, Gerich JE (2002) Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab 282: E419–427. - PubMed
-
- Singhal P, Caumo A, Carey PE, Cobelli C, Taylor R (2002) Regulation of endogenous glucose production after a mixed meal in type 2 diabetes. Am J Physiol Endocrinol Metab 283: E275–283. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

