Reorganization of the connectivity of cortical field DZ in congenitally deaf cat
- PMID: 23593166
- PMCID: PMC3625188
- DOI: 10.1371/journal.pone.0060093
Reorganization of the connectivity of cortical field DZ in congenitally deaf cat
Abstract
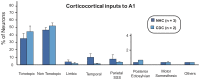
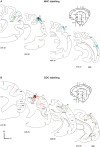
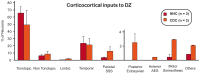
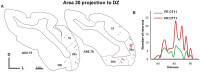
Psychophysics and brain imaging studies in deaf patients have revealed a functional crossmodal reorganization that affects the remaining sensory modalities. Similarly, the congenital deaf cat (CDC) shows supra-normal visual skills that are supported by specific auditory fields (DZ-dorsal zone and P-posterior auditory cortex) but not the primary auditory cortex (A1). To assess the functional reorganization observed in deafness we analyzed the connectivity pattern of the auditory cortex by means of injections of anatomical tracers in DZ and A1 in both congenital deaf and normally hearing cats. A quantitative analysis of the distribution of the projecting neurons revealed the presence of non-auditory inputs to both A1 and DZ of the CDC which were not observed in the hearing cats. Firstly, some visual (areas 19/20) and somatosensory (SIV) areas were projecting toward DZ of the CDC but not in the control. Secondly, A1 of the deaf cat received a weak projection from the visual lateral posterior nuclei (LP). Most of these abnormal projections to A1 and DZ represent only a small fraction of the normal inputs to these areas. In addition, most of the afferents to DZ and A1 appeared normal in terms of areal specificity and strength of projection, with preserved but smeared nucleotopic gradient of A1 in CDCs. In conclusion, while the abnormal projections revealed in the CDC can participate in the crossmodal compensatory mechanisms, the observation of a limited reorganization of the connectivity pattern of the CDC implies that functional reorganization in congenital deafness is further supported also by normal cortico-cortical connectivity.
Conflict of interest statement
Figures

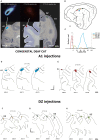


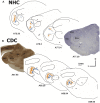
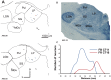
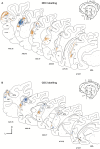
References
-
- Rauschecker JP (1995) Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci 18: 36–43. - PubMed
-
- Collignon O, Champoux F, Voss P, Lepore F (2011) Sensory rehabilitation in the plastic brain. Prog Brain Res 191: 211–231. - PubMed
-
- Röder B, Rosler F (2004) Compensatory plasticity as a consequence of sensory loss. In: Calvert G, Spence C, Stein BE, editors. The handbook of multisensory processes. Cambridge, Mass.: MIT Press. 719–747.
-
- Pavani F, Bottari D (2011) Visual abilities in individuals with profound deafness: A critical review. In: Murray MM, Wallace MN, editors. Frontiers in the neural bases of multisensory processes. Boca Raton: CRC Press. in press. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

