Amiloride but not memantine reduces neurodegeneration, seizures and myoclonic jerks in rats with cardiac arrest-induced global cerebral hypoxia and reperfusion
- PMID: 23593189
- PMCID: PMC3620224
- DOI: 10.1371/journal.pone.0060309
Amiloride but not memantine reduces neurodegeneration, seizures and myoclonic jerks in rats with cardiac arrest-induced global cerebral hypoxia and reperfusion
Abstract
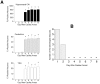
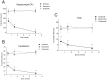
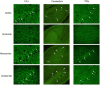
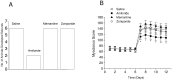
It has been reported that both activation of N-methyl-D-aspartate receptors and acid-sensing ion channels during cerebral ischemic insult contributed to brain injury. But which of these two molecular targets plays a more pivotal role in hypoxia-induced brain injury during ischemia is not known. In this study, the neuroprotective effects of an acid-sensing cation channel blocker and an N-methyl-D-aspartate receptor blocker were evaluated in a rat model of cardiac arrest-induced cerebral hypoxia. We found that intracisternal injection of amiloride, an acid-sensing ion channel blocker, dose-dependently reduced cerebral hypoxia-induced neurodegeneration, seizures, and audiogenic myoclonic jerks. In contrast, intracisternal injection of memantine, a selective uncompetitive N-methyl-D-aspartate receptor blocker, had no significant effect on cerebral hypoxia-induced neurodegeneration, seizure and audiogenic myoclonic jerks. Intracisternal injection of zoniporide, a specific sodium-hydrogen exchanger inhibitor, before cardiac arrest-induced cerebral hypoxia, also did not reduce cerebral hypoxia-induced neurodegeneration, seizures and myoclonic jerks. These results suggest that acid-sensing ion channels play a more pivotal role than N-methyl-D-aspartate receptors in mediating cerebral hypoxia-induced brain injury during ischemic insult.
Conflict of interest statement
Figures
Similar articles
-
Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke?Prog Neurobiol. 2014 Apr;115:189-209. doi: 10.1016/j.pneurobio.2013.12.008. Epub 2014 Jan 24. Prog Neurobiol. 2014. PMID: 24467911 Free PMC article. Review.
-
Memantine exacerbates myoclonic jerks in a rat model of posthypoxic myoclonus.Brain Res. 2010 Jul 9;1343:194-8. doi: 10.1016/j.brainres.2010.04.058. Epub 2010 Apr 29. Brain Res. 2010. PMID: 20434435
-
Memantine delayed N-methyl-D-aspartate -induced convulsions in neonatal rats.Fundam Clin Pharmacol. 2015 Feb;29(1):72-8. doi: 10.1111/fcp.12090. Epub 2014 Dec 2. Fundam Clin Pharmacol. 2015. PMID: 25196574
-
NMDA receptor-mediated excitotoxicity contributes to the cerebral hypoxic injury of a rat model of posthypoxic myoclonus.Brain Res. 2007 Feb 16;1133(1):209-15. doi: 10.1016/j.brainres.2006.11.076. Epub 2006 Dec 28. Brain Res. 2007. PMID: 17196560
-
Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a "two-hit" model.Crit Care. 2017 Apr 13;21(1):90. doi: 10.1186/s13054-017-1670-9. Crit Care. 2017. PMID: 28403909 Free PMC article. Review.
Cited by
-
Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke?Prog Neurobiol. 2014 Apr;115:189-209. doi: 10.1016/j.pneurobio.2013.12.008. Epub 2014 Jan 24. Prog Neurobiol. 2014. PMID: 24467911 Free PMC article. Review.
-
Enhanced harm detection following maternal separation: Transgenerational transmission and reversibility by inhaled amiloride.Sci Adv. 2023 Oct 6;9(40):eadi8750. doi: 10.1126/sciadv.adi8750. Epub 2023 Oct 4. Sci Adv. 2023. PMID: 37792939 Free PMC article.
-
Synthetic analogues of memantine as neuroprotective and influenza viral inhibitors: in vitro and physicochemical studies.Amino Acids. 2020 Dec;52(11-12):1559-1580. doi: 10.1007/s00726-020-02914-4. Epub 2020 Nov 15. Amino Acids. 2020. PMID: 33191465
-
Amiloride suppresses pilocarpine-induced seizures via ASICs other than NHE in rats.Int J Clin Exp Pathol. 2015 Nov 1;8(11):14507-13. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26823770 Free PMC article.
-
Altered Expression Pattern of Acid-Sensing Ion Channel Isoforms in Piriform Cortex After Seizures.Mol Neurobiol. 2016 Apr;53(3):1782-1793. doi: 10.1007/s12035-015-9130-5. Epub 2015 Mar 7. Mol Neurobiol. 2016. PMID: 25744567
References
-
- Olney JW (1969) Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164: 719–721. - PubMed
-
- Olney JW, Sharpe LG (1969) Brain lesions in an infant rhesus monkey treated with monsodium glutamate. Science 166: 386–388. - PubMed
-
- Goldberg MP, Weiss JH, Pham PC, Choi DW (1987) N-methyl-D-aspartate receptors mediate hypoxic neuronal injury in cortical culture. J Pharmacol Exp Ther 243: 784–791. - PubMed
-
- Choi DW (1990) Rothman (1990) The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu.Rev. Neurosci13: 171–182. - PubMed
-
- Simon RP, Swan JH, Griffiths T, Meldrum BS (1984) Blockade of N-methyl-D-aspartate receptors may protect against ischemic damage in the brain. Science 226: 850–852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

