Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with non-taxane-containing palliative chemotherapy for gastric carcinoma: a systematic review and meta-analysis
- PMID: 23593191
- PMCID: PMC3617226
- DOI: 10.1371/journal.pone.0060320
Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with non-taxane-containing palliative chemotherapy for gastric carcinoma: a systematic review and meta-analysis
Abstract
Background: Gastric carcinoma (GC) is one of the highest cancer-mortality diseases with a high incidence rate in Asia. For surgically unfit but medically fit patients, palliative chemotherapy is the main treatment. The chemotherapy regimen of docetaxel, cisplatin and 5-fluorouracil (DCF) has been used to treat the advanced stage or metastatic GC. It is necessary to compare effectiveness and toxicities of DCF regimen with non-taxane-containing palliative chemotherapy for GC.
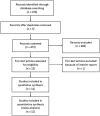
Methods: PubMed, EmBase, Cochrane Central Register of Controlled Trials and China National Knowledge Infrastructure databases were searched to select relative randomized controlled trials (RCTs) comparing DCF to non-taxane-containing chemotherapy for patients with palliatively resected, unresectable, recurrent or metastatic GC. Primary outcome measures were 1-year and 2-year overall survival (OS) rates. Secondary outcome measures were median survival time (MST), median time to progression (TTP), response rate and toxicities.
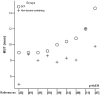
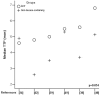
Results: Twelve RCTs were eligible and 1089 patients were analyzed totally (549 in DCF and 540 in control). DCF regimen increased partial response rate (38.8% vs 27.9%, p = 0.0003) and reduced progressive disease rate (18.9% vs 33.3%, p = 0.0005) compared to control regimen. Significant improvement of 2-year OS rate was found in DCF regimen (RR = 2.03, p = 0.006), but not of 1-year OS rate (RR = 1.22, p = 0.08). MST was significantly prolonged by DCF regimen (p = 0.039), but not median TTP (p = 0.054). Both 1-year OS rate and median TTP had a trend of prolongation by DCF regimen. Chemotherapy-related mortality was comparable (RR = 1.23, p = 0.49) in both regimens. In grade I-IV toxicities, DCF regimen showed a major raise of febrile neutropenia (RR = 2.33, p<0.0001) and minor raises of leucopenia (RR = 1.25, p<0.00001), neutropenia (RR = 1.19, p<0.00001), and diarrhea (RR = 1.59, p<0.00001), while in other toxicities there were no significant differences.
Conclusion: DCF regimen has better response than non-taxane containing regimen and could potentially improve the survival outcomes. The chemotherapy-related toxicity of DCF regimen is acceptable to some extent.
Conflict of interest statement
Figures
References
-
- Wagner AD, Wedding U (2009) Advances in the pharmacological treatment of gastro-oesophageal cancer. Drugs Aging 26: 627–646. - PubMed
-
- Bittoni A, Maccaroni E, Scartozzi M, Berardi R, Cascinu S (2010) Chemotherapy for locally advanced and metastatic gastric cancer: state of the art and future perspectives. Eur Rev Med Pharmacol Sci 14: 309–314. - PubMed
-
- Catalano V, Labianca R, Beretta GD, Gatta G, De Braud F, et al. (2009) Gastric cancer. Crit Rev Oncol Hematol 71: 127–164. - PubMed
-
- Ajani JA (1998) Chemotherapy for gastric carcinoma: new and old options. Oncology (Williston Park) 12(10 Suppl 7 44–47. - PubMed
-
- Tsai JY, Safran H (2003) Status of treatment for advanced gastric carcinoma. Curr Oncol Rep 5: 210–218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous