Pro-asthmatic cytokines regulate unliganded and ligand-dependent glucocorticoid receptor signaling in airway smooth muscle
- PMID: 23593222
- PMCID: PMC3617099
- DOI: 10.1371/journal.pone.0060452
Pro-asthmatic cytokines regulate unliganded and ligand-dependent glucocorticoid receptor signaling in airway smooth muscle
Abstract
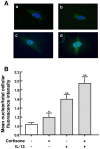
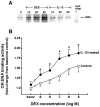
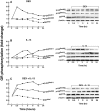
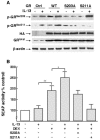
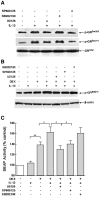
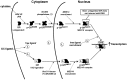
To elucidate the regulation of glucocorticoid receptor (GR) signaling under pro-asthmatic conditions, cultured human airway smooth muscle (HASM) cells were treated with proinflammatory cytokines or GR ligands alone and in combination, and then examined for induced changes in ligand-dependent and -independent GR activation and downstream signaling events. Ligand stimulation with either cortisone or dexamethsone (DEX) acutely elicited GR translocation to the nucleus and, comparably, ligand-independent stimulation either with the Th2 cytokine, IL-13, or the pleiotropic cytokine combination, IL-1β/TNFα, also acutely evoked GR translocation. The latter response was potentiated by combined exposure of cells to GR ligand and cytokine. Similarly, treatment with either DEX or IL-13 alone induced GR phosphorylation at its serine-211 residue (GR(Ser211)), denoting its activated state, and combined treatment with DEX+IL-13 elicited heightened and sustained GR(Ser211) phosphorylation. Interestingly, the above ligand-independent GR responses to IL-13 alone were not associated with downstream GR binding to its consensus DNA sequence or GR transactivation, whereas both DEX-induced GR:DNA binding and transcriptional activity were significantly heightened in the presence of IL-13, coupled to increased recruitment of the transcriptional co-factor, MED14. The stimulated GR signaling responses to DEX were prevented in IL-13-exposed cells wherein GR(Ser211) phosphorylation was suppressed either by transfection with specific serine phosphorylation-deficient mutant GRs or treatment with inhibitors of the MAPKs, ERK1/2 and JNK. Collectively, these novel data highlight a heretofore-unidentified homeostatic mechanism in HASM cells that involves pro-asthmatic cytokine-driven, MAPK-mediated, non-ligand-dependent GR activation that confers heightened glucocorticoid ligand-stimulated GR signaling. These findings raise the consideration that perturbations in this homeostatic cytokine-driven GR signaling mechanism may be responsible, at least in part, for the insensirtivity to glucocorticoid therapy that is commonly seen in individuals with severe asthma.
Conflict of interest statement
Figures


References
-
- Barnes PJ (1998) Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci 94: 557–572. - PubMed
-
- Webster JC, Cidlowski JA (1999) Mechanisms of glucocorticoid-receptor-mediated repression of gene expression. Trends Endocrinol Metab 10: 396–402. - PubMed
-
- Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP (2003) Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol 85: 457–467. - PubMed
-
- Chapman KE, Coutinho A, Gray M, Gilmour JS, Savill JS, et al. (2006) Local Amplification of Glucocorticoids by 11β-Hydroxysteroid Dehydrogenase Type 1 and Its Role in the Inflammatory Response. Ann NY Acad Sci 1088: 265–273. - PubMed
-
- Cooper MS, Bujalska I, Rabbitt E, Walker EA, Bland R, et al. (2001) Modulation of 11beta-hydroxysteroid dehydrogenase isozymes by proinflammatory cytokines in osteoblasts: an autocrine switch from glucocorticoid inactivation to activation. J Bone Miner Res 16: 1037–1044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

