Reduced androgen receptor expression accelerates the onset of ERBB2 induced breast tumors in female mice
- PMID: 23593223
- PMCID: PMC3620158
- DOI: 10.1371/journal.pone.0060455
Reduced androgen receptor expression accelerates the onset of ERBB2 induced breast tumors in female mice
Abstract
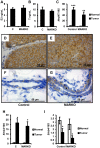
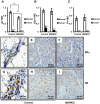
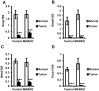
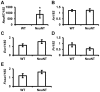
Androgen receptor (AR) is commonly expressed in both the epithelium of normal mammary glands and in breast cancers. AR expression in breast cancers is independent of estrogen receptor alpha (ERα) status and is frequently associated with overexpression of the ERBB2 oncogene. AR signaling effects on breast cancer progression may depend on ERα and ERBB2 status. Up to 30% of human breast cancers are driven by overactive ERBB2 signaling and it is not clear whether AR expression affects any steps of tumor progression in this cohort of patients. To test this, we generated mammary specific Ar depleted mice (MARKO) by combining the floxed allele of Ar with the MMTV-cre transgene on an MMTV-NeuNT background and compared them to littermate MMTV-NeuNT, Ar(fl)/+ control females. Heterozygous MARKO females displayed reduced levels of AR in mammary glands with mosaic AR expression in ductal epithelium. The loss of AR dramatically accelerated the onset of MMTV-NeuNT tumors in female MARKO mice. In this report we show that accelerated MMTV-NeuNT-dependent tumorigenesis is due specifically to the loss of AR, as hormonal levels, estrogen and progesterone receptors expression, and MMTV-NeuNT expression were similar between MARKO and control groups. MMTV-NeuNT induced tumors in both cohorts displayed distinct loss of AR in addition to ERα, PR, and the pioneer factor FOXA1. Erbb3 mRNA levels were significantly elevated in tumors in comparison to normal mammary glands. Thus the loss of AR in mouse mammary epithelium accelerates malignant transformation rather than the rate of tumorigenesis.
Conflict of interest statement
Figures



Similar articles
-
Accelerated mammary maturation and differentiation, and delayed MMTVneu-induced tumorigenesis of K303R mutant ERalpha transgenic mice.Oncogene. 2009 Sep 10;28(36):3177-87. doi: 10.1038/onc.2009.174. Epub 2009 Jun 29. Oncogene. 2009. PMID: 19561644 Free PMC article.
-
Sustained trophism of the mammary gland is sufficient to accelerate and synchronize development of ErbB2/Neu-induced tumors.Oncogene. 2006 Jun 1;25(23):3325-34. doi: 10.1038/sj.onc.1209365. Epub 2006 Jan 23. Oncogene. 2006. PMID: 16434967 Free PMC article.
-
USP22 overexpression fails to augment tumor formation in MMTV-ERBB2 mice but loss of function impacts MMTV promoter activity.PLoS One. 2024 Jan 18;19(1):e0290837. doi: 10.1371/journal.pone.0290837. eCollection 2024. PLoS One. 2024. PMID: 38236941 Free PMC article.
-
Developmental timing of activated erbB2 expression plays a critical role in the induction of mammary tumors.Cell Cycle. 2004 Sep;3(9):1111-3. Epub 2004 Sep 1. Cell Cycle. 2004. PMID: 15326381 Review.
-
ErbB2 activation and signal transduction in normal and malignant mammary cells.J Mammary Gland Biol Neoplasia. 1996 Apr;1(2):199-206. doi: 10.1007/BF02013643. J Mammary Gland Biol Neoplasia. 1996. PMID: 10887493 Review.
Cited by
-
Advances in Rodent Models for Breast Cancer Formation, Progression, and Therapeutic Testing.Front Oncol. 2021 Mar 26;11:593337. doi: 10.3389/fonc.2021.593337. eCollection 2021. Front Oncol. 2021. PMID: 33842308 Free PMC article. Review.
-
Inositol polyphosphate 4-phosphatase type II regulation of androgen receptor activity.Oncogene. 2019 Feb;38(7):1121-1135. doi: 10.1038/s41388-018-0498-3. Epub 2018 Sep 18. Oncogene. 2019. PMID: 30228349 Free PMC article.
-
Deletion of inositol polyphosphate 4-phosphatase type-II B affects spermatogenesis in mice.PLoS One. 2020 May 15;15(5):e0233163. doi: 10.1371/journal.pone.0233163. eCollection 2020. PLoS One. 2020. PMID: 32413098 Free PMC article.
-
New Advances in Targeted Therapy of HER2-Negative Breast Cancer.Front Oncol. 2022 Mar 4;12:828438. doi: 10.3389/fonc.2022.828438. eCollection 2022. Front Oncol. 2022. PMID: 35311116 Free PMC article. Review.
-
Sputum Detection of Predisposing Genetic Mutations in Women with Pulmonary Nontuberculous Mycobacterial Disease.Sci Rep. 2018 Jul 27;8(1):11336. doi: 10.1038/s41598-018-29471-x. Sci Rep. 2018. PMID: 30054559 Free PMC article.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, et al. (1987) Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235: 177–182. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, et al. (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244: 707–712. - PubMed
-
- Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, et al. (2008) A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 68: 5878–5887. - PubMed
-
- Bouchard L, Lamarre L, Tremblay PJ, Jolicoeur P (1989) Stochastic appearance of mammary tumors in transgenic mice carrying the MMTV/c-neu oncogene. Cell 57: 931–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

