Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study
- PMID: 23593236
- PMCID: PMC3622671
- DOI: 10.1371/journal.pone.0060537
Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study
Erratum in
- PLoS One. 2013;8(6). 10.1371/annotation/1c75cd5d-9dde-4ace-8524-a4980745e804
Abstract
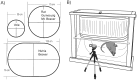
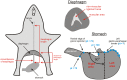
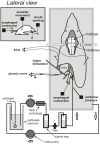
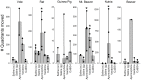
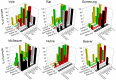
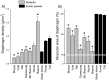
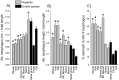
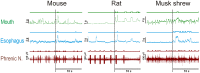
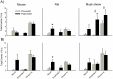
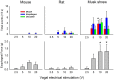
The vomiting (emetic) reflex is documented in numerous mammalian species, including primates and carnivores, yet laboratory rats and mice appear to lack this response. It is unclear whether these rodents do not vomit because of anatomical constraints (e.g., a relatively long abdominal esophagus) or lack of key neural circuits. Moreover, it is unknown whether laboratory rodents are representative of Rodentia with regards to this reflex. Here we conducted behavioral testing of members of all three major groups of Rodentia; mouse-related (rat, mouse, vole, beaver), Ctenohystrica (guinea pig, nutria), and squirrel-related (mountain beaver) species. Prototypical emetic agents, apomorphine (sc), veratrine (sc), and copper sulfate (ig), failed to produce either retching or vomiting in these species (although other behavioral effects, e.g., locomotion, were noted). These rodents also had anatomical constraints, which could limit the efficiency of vomiting should it be attempted, including reduced muscularity of the diaphragm and stomach geometry that is not well structured for moving contents towards the esophagus compared to species that can vomit (cat, ferret, and musk shrew). Lastly, an in situ brainstem preparation was used to make sensitive measures of mouth, esophagus, and shoulder muscular movements, and phrenic nerve activity-key features of emetic episodes. Laboratory mice and rats failed to display any of the common coordinated actions of these indices after typical emetic stimulation (resiniferatoxin and vagal afferent stimulation) compared to musk shrews. Overall the results suggest that the inability to vomit is a general property of Rodentia and that an absent brainstem neurological component is the most likely cause. The implications of these findings for the utility of rodents as models in the area of emesis research are discussed.
Conflict of interest statement
Figures



Similar articles
-
The involvement of TRPV1 in emesis and anti-emesis.Temperature (Austin). 2015 May 21;2(2):258-76. doi: 10.1080/23328940.2015.1043042. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227028 Free PMC article. Review.
-
An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing.Exp Physiol. 2002 Sep;87(5):563-74. doi: 10.1113/eph8702424. Exp Physiol. 2002. PMID: 12481931
-
[Unique structure of the esophago-gastric junction of the house musk shrew (Suncus murinus)].Kaibogaku Zasshi. 2004 Dec;79(4):121-9. Kaibogaku Zasshi. 2004. PMID: 15678992 Japanese.
-
The development of the emetic reflex in the house musk shrew, Suncus murinus.Brain Res Dev Brain Res. 2000 May 11;121(1):29-34. doi: 10.1016/s0165-3806(00)00022-5. Brain Res Dev Brain Res. 2000. PMID: 10837890
-
The medical implications of gastrointestinal vagal afferent pathways in nausea and vomiting.Curr Pharm Des. 2014;20(16):2703-12. doi: 10.2174/13816128113199990568. Curr Pharm Des. 2014. PMID: 23886386 Review.
Cited by
-
Odor-Induced Vomiting Is Combinatorially Triggered by Palp Olfactory Receptor Neurons That Project to the Lobus Glomerulatus in Locust Brain.Front Physiol. 2022 Apr 20;13:855522. doi: 10.3389/fphys.2022.855522. eCollection 2022. Front Physiol. 2022. PMID: 35514359 Free PMC article.
-
Mouse Anesthesia: The Art and Science.ILAR J. 2021 Dec 31;62(1-2):238-273. doi: 10.1093/ilar/ilab016. ILAR J. 2021. PMID: 34180990 Free PMC article. Review.
-
Antiemetic Effects of Cannabinoid Agonists in Nonhuman Primates.J Pharmacol Exp Ther. 2020 Sep;374(3):462-468. doi: 10.1124/jpet.120.265710. Epub 2020 Jun 19. J Pharmacol Exp Ther. 2020. PMID: 32561684 Free PMC article.
-
Mechanisms and prevention of acid reflux induced laryngospasm in seizing rats.Epilepsy Behav. 2020 Oct;111:107188. doi: 10.1016/j.yebeh.2020.107188. Epub 2020 Jun 12. Epilepsy Behav. 2020. PMID: 32540771 Free PMC article.
-
The involvement of TRPV1 in emesis and anti-emesis.Temperature (Austin). 2015 May 21;2(2):258-76. doi: 10.1080/23328940.2015.1043042. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227028 Free PMC article. Review.
References
-
- Florczyk AP, Schurig JE, Bradner WT (1982) Cisplatin-induced emesis in the Ferret: a new animal model. Cancer Treat Rep 66: 187–189. - PubMed
-
- Papp RH, Hawkins HB, Share NN, Wang SC (1966) Emesis induced by the intracerebroventricular administration of hydergine and mechlorethamine hydrochloride. J Pharmacol Exp Ther 154: 333–338. - PubMed
-
- Gylys JA, Doran KM, Buyniski JP (1979) Antagonism of cisplatin induced emesis in the dog. Res Commun Chem Pathol Pharmacol 23: 61–68. - PubMed
-
- Schurig JE, Florczyk AP, Rose WC, Bradner WT (1982) Antiemetic activity of butorphanol against cisplatin-induced emesis in ferrets and dogs. Cancer Treat Rep 66: 1831–1835. - PubMed
-
- Costello DJ, Borison HL (1977) Naloxone antagonizes narcotic self blockade of emesis in the cat. J Pharmacol Exp Ther 203: 222–230. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

