Purification, gene cloning, and biochemical characterization of a β-glucosidase capable of hydrolyzing sesaminol triglucoside from Paenibacillus sp. KB0549
- PMID: 23593237
- PMCID: PMC3622683
- DOI: 10.1371/journal.pone.0060538
Purification, gene cloning, and biochemical characterization of a β-glucosidase capable of hydrolyzing sesaminol triglucoside from Paenibacillus sp. KB0549
Abstract
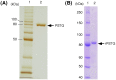
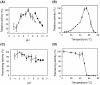
The triglucoside of sesaminol, i.e., 2,6-O-di(β-D-glucopyranosyl)-β-D- glucopyranosylsesaminol (STG), occurs abundantly in sesame seeds and sesame oil cake and serves as an inexpensive source for the industrial production of sesaminol, an anti-oxidant that displays a number of bioactivities beneficial to human health. However, STG has been shown to be highly resistant to the action of β-glucosidases, in part due to its branched-chain glycon structure, and these circumstances hampered the efficient utilization of STG. We found that a strain (KB0549) of the genus Paenibacillus produced a novel enzyme capable of efficiently hydrolyzing STG. This enzyme, termed PSTG, was a tetrameric protein consisting of identical subunits with an approximate molecular mass of 80 kDa. The PSTG gene was cloned on the basis of the partial amino acid sequences of the purified enzyme. Sequence comparison showed that the enzyme belonged to the glycoside hydrolase family 3, with significant similarities to the Paenibacillus glucocerebrosidase (63% identity) and to Bgl3B of Thermotoga neapolitana (37% identity). The recombinant enzyme (rPSTG) was highly specific for β-glucosidic linkage, and k cat and k cat/K m values for the rPSTG-catalyzed hydrolysis of p-nitrophenyl-β-glucopyraniside at 37°C and pH 6.5 were 44 s(-1) and 426 s(-1) mM(-1), respectively. The specificity analyses also revealed that the enzyme acted more efficiently on sophorose than on cellobiose and gentiobiose. Thus, rPSTG is the first example of a β-glucosidase with higher reactivity for β-1,2-glucosidic linkage than for β-1,4- and β-1,6-glucosidic linkages, as far as could be ascertained. This unique specificity is, at least in part, responsible for the enzyme's ability to efficiently decompose STG.
Conflict of interest statement
Figures


References
-
- Fukuda Y, Nagata M, Osawa T, Namiki M (1986) Contribution of lignan analogs to antioxidative activity of refined unroasted sesame seed oil. Journal of the American Oil Chemists’ Society 63: 1027–1031.
-
- Kumazawa S, Koike M, Usui Y, Nakayama T, Fukuda Y (2003) Isolation of sesaminols as antioxidative components from roasted sesame seed oil. Journal of Oleo Science 52: 303–307.
-
- Kang MH, Katsuzaki H, Osawa T (1998) Inhibition of 2,2′-azobis(2,4-dimethylvaleronitrile)-induced lipid peroxidation by sesaminols. Lipids 33: 1031–1036. - PubMed
-
- Kang MH, Naito M, Sakai K, Uchida K, Osawa T (2000) Mode of action of sesame lignans in protecting low-density lipoprotein against oxidative damage in vitro . Life Sciences 66: 161–171. - PubMed
-
- Miyahara Y, Hibasami H, Katsuzaki H, Imai K, Osawa T, et al. (2001) Sesaminol from sesame seed induces apoptosis in human lymphoid leukemia Molt 4B cells. International Journal of Molecular Medicine 7: 485–488. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

