Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury
- PMID: 23593240
- PMCID: PMC3623813
- DOI: 10.1371/journal.pone.0060546
Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury
Abstract
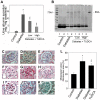
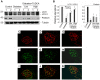
Despite the recent attention focused on the important role of autophagy in maintaining podocyte homeostasis, little is known about the changes and mechanisms of autophagy in podocyte dysfunction under diabetic condition. In this study, we investigated the role of autophagy in podocyte biology and its involvement in the pathogenesis of diabetic nephropathy. Podocytes had a high basal level of autophagy. And basal autophagy inhibition either by 3-methyladenenine (3-MA) or by Beclin-1 siRNA was detrimental to its architectural structure. However, under diabetic condition in vivo and under high glucose conditions in vitro, high basal level of autophagy in podocytes became defective and defective autophagy facilitated the podocyte injury. Since the dynamics of endoplasmic reticulum(ER) seemed to play a vital role in regulating the autophagic flux, the results that Salubrinal/Tauroursodeoxycholic acid (TUDCA) could restore defective autophagy further indicated that the evolution of autophagy may be mediated by the changes of cytoprotective output in the ER stress. Finally, we demonstrated in vivo that the autophagy of podocyte was inhibited under diabetic status and TUDCA could improve defective autophagy. Taken together, these data suggested that autophagy might be interrupted due to the failure of ER cytoprotective capacity upon high glucose induced unmitigated stress, and the defective autophagy might accelerate the irreparable progression of diabetic nephropathy.
Conflict of interest statement
Figures
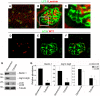



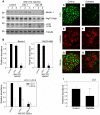
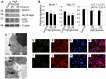

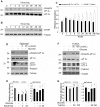
References
-
- Pavenstadt H, Kriz W, Kretzler M (2003) Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307. - PubMed
-
- Wolf G, Chen S, Ziyadeh FN (2005) From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 54: 1626–1634. - PubMed
-
- Quaggin SE (2002) Transcriptional regulation of podocyte specification and differentiation. Microsc Res Tech 57: 208–211. - PubMed
-
- Kobayashi N (2002) Mechanism of the process formation; podocytes vs. neurons. Microsc Res Tech 57: 217–223. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

