Age-dependent impairment of eyeblink conditioning in prion protein-deficient mice
- PMID: 23593266
- PMCID: PMC3622692
- DOI: 10.1371/journal.pone.0060627
Age-dependent impairment of eyeblink conditioning in prion protein-deficient mice
Abstract
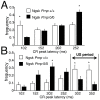
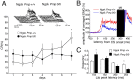
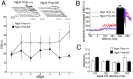
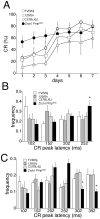
Mice lacking the prion protein (PrP(C)) gene (Prnp), Ngsk Prnp (0/0) mice, show late-onset cerebellar Purkinje cell (PC) degeneration because of ectopic overexpression of PrP(C)-like protein (PrPLP/Dpl). Because PrP(C) is highly expressed in cerebellar neurons (including PCs and granule cells), it may be involved in cerebellar synaptic function and cerebellar cognitive function. However, no studies have been conducted to investigate the possible involvement of PrP(C) and/or PrPLP/Dpl in cerebellum-dependent discrete motor learning. Therefore, the present cross-sectional study was designed to examine cerebellum-dependent delay eyeblink conditioning in Ngsk Prnp (0/0) mice in adulthood (16, 40, and 60 weeks of age). The aims of the present study were two-fold: (1) to examine the role of PrP(C) and/or PrPLP/Dpl in cerebellum-dependent motor learning and (2) to confirm the age-related deterioration of eyeblink conditioning in Ngsk Prnp (0/0) mice as an animal model of progressive cerebellar degeneration. Ngsk Prnp (0/0) mice aged 16 weeks exhibited intact acquisition of conditioned eyeblink responses (CRs), although the CR timing was altered. The same result was observed in another line of PrP(c)-deficient mice, ZrchI PrnP (0/0) mice. However, at 40 weeks of age, CR incidence impairment was observed in Ngsk Prnp (0/0) mice. Furthermore, Ngsk Prnp (0/0) mice aged 60 weeks showed more significantly impaired CR acquisition than Ngsk Prnp (0/0) mice aged 40 weeks, indicating the temporal correlation between cerebellar PC degeneration and motor learning deficits. Our findings indicate the importance of the cerebellar cortex in delay eyeblink conditioning and suggest an important physiological role of prion protein in cerebellar motor learning.
Conflict of interest statement
Figures

Similar articles
-
Impairment of cerebellar long-term depression and GABAergic transmission in prion protein deficient mice ectopically expressing PrPLP/Dpl.Sci Rep. 2020 Sep 28;10(1):15900. doi: 10.1038/s41598-020-72753-6. Sci Rep. 2020. PMID: 32985542 Free PMC article.
-
Abnormal activation of glial cells in the brains of prion protein-deficient mice ectopically expressing prion protein-like protein, PrPLP/Dpl.Mol Med. 2001 Dec;7(12):803-9. Mol Med. 2001. PMID: 11844868 Free PMC article.
-
Deletion of N-terminal residues 23-88 from prion protein (PrP) abrogates the potential to rescue PrP-deficient mice from PrP-like protein/doppel-induced Neurodegeneration.J Biol Chem. 2003 Aug 1;278(31):28944-9. doi: 10.1074/jbc.M303655200. Epub 2003 May 19. J Biol Chem. 2003. PMID: 12759361
-
The prion gene complex encoding PrP(C) and Doppel: insights from mutational analysis.Gene. 2001 Sep 5;275(1):1-18. doi: 10.1016/s0378-1119(01)00627-8. Gene. 2001. PMID: 11574147 Review.
-
Biology of the prion gene complex.Biochem Cell Biol. 2001;79(5):613-28. doi: 10.1139/o01-142. Biochem Cell Biol. 2001. PMID: 11716303 Review.
Cited by
-
Implicit Memory in Monkeys: Development of a Delay Eyeblink Conditioning System with Parallel Electromyographic and High-Speed Video Measurements.PLoS One. 2015 Jun 12;10(6):e0129828. doi: 10.1371/journal.pone.0129828. eCollection 2015. PLoS One. 2015. PMID: 26068663 Free PMC article.
-
RNA-binding proteins associated molecular mechanisms of motor neuron degeneration pathogenesis.Mol Biotechnol. 2014 Sep;56(9):779-86. doi: 10.1007/s12033-014-9785-6. Mol Biotechnol. 2014. PMID: 25052333 Review.
-
Functional maintenance of calcium store by ShcB adaptor protein in cerebellar Purkinje cells.Sci Rep. 2020 Sep 2;10(1):14475. doi: 10.1038/s41598-020-71414-y. Sci Rep. 2020. PMID: 32879382 Free PMC article.
-
Dataset of eyeblink conditioning in mice treated with the selective mGluR1 antagonist JNJ16259685.Data Brief. 2023 Jan 25;47:108935. doi: 10.1016/j.dib.2023.108935. eCollection 2023 Apr. Data Brief. 2023. PMID: 36798600 Free PMC article.
-
Assessment of retention and attenuation of motor-learning memory by repeated rotor-rod analyses.Sci Rep. 2024 Dec 28;14(1):31003. doi: 10.1038/s41598-024-82108-0. Sci Rep. 2024. PMID: 39730861 Free PMC article.
References
-
- Collinge J (1999) Variant Creutzfeldt-Jakob disease. Lancet 354: 317–323. - PubMed
-
- Gajdusek DC (1977) Unconventional viruses and the origin and disappearance of kuru. Science 197: 943–960. - PubMed
-
- Horwich AL, Weissman JS (1997) Deadly conformations–protein misfolding in prion disease. Cell 89: 499–510. - PubMed
-
- Prusiner SB (1997) Prion diseases and the BSE crisis. Science 278: 245–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

