Post-hypoxic recovery of respiratory rhythm generation is gender dependent
- PMID: 23593283
- PMCID: PMC3620234
- DOI: 10.1371/journal.pone.0060695
Post-hypoxic recovery of respiratory rhythm generation is gender dependent
Abstract
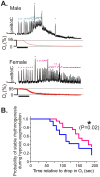
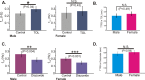
The preBötzinger complex (preBötC) is a critical neuronal network for the generation of breathing. Lesioning the preBötC abolishes respiration, while when isolated in vitro, the preBötC continues to generate respiratory rhythmic activity. Although several factors influence rhythmogenesis from this network, little is known about how gender may affect preBötC function. This study examines the influence of gender on respiratory activity and in vitro rhythmogenesis from the preBötC. Recordings of respiratory activity from neonatal mice (P10-13) show that sustained post-hypoxic depression occurs with greater frequency in males compared to females. Moreover, extracellular population recordings from the preBötC in neonatal brainstem slices (P10-13) reveal that the time to the first inspiratory burst following reoxygenation (TTFB) is significantly delayed in male rhythmogenesis when compared to the female rhythms. Altering activity of ATP sensitive potassium channels (KATP) with either the agonist, diazoxide, or the antagonist, tolbutamide, eliminates differences in TTFB. By contrast, glucose supplementation improves post-hypoxic recovery of female but not male rhythmogenesis. We conclude that post-hypoxic recovery of respiration is gender dependent, which is, in part, centrally manifested at the level of the preBötC. Moreover, these findings provide potential insight into the basis of increased male vulnerability in a variety of conditions such as Sudden Infant Death Syndrome (SIDS).
Conflict of interest statement
Figures







Similar articles
-
Dendritic A-Current in Rhythmically Active PreBötzinger Complex Neurons in Organotypic Cultures from Newborn Mice.J Neurosci. 2018 Mar 21;38(12):3039-3049. doi: 10.1523/JNEUROSCI.3342-17.2018. Epub 2018 Feb 19. J Neurosci. 2018. PMID: 29459371 Free PMC article.
-
Carbenoxolone induced depression of rhythmogenesis in the pre-Bötzinger Complex.BMC Neurosci. 2008 May 23;9:46. doi: 10.1186/1471-2202-9-46. BMC Neurosci. 2008. PMID: 18500991 Free PMC article.
-
Optogenetic excitation of preBötzinger complex neurons potently drives inspiratory activity in vivo.J Physiol. 2015 Aug 15;593(16):3673-92. doi: 10.1113/JP270471. Epub 2015 Jul 14. J Physiol. 2015. PMID: 26010654 Free PMC article.
-
Respiratory rhythm generation, hypoxia, and oxidative stress-Implications for development.Respir Physiol Neurobiol. 2019 Dec;270:103259. doi: 10.1016/j.resp.2019.103259. Epub 2019 Jul 29. Respir Physiol Neurobiol. 2019. PMID: 31369874 Free PMC article. Review.
-
Pre-Bötzinger complex: Generation and modulation of respiratory rhythm.Neurologia (Engl Ed). 2019 Sep;34(7):461-468. doi: 10.1016/j.nrl.2016.05.011. Epub 2016 Jul 18. Neurologia (Engl Ed). 2019. PMID: 27443242 Review. English, Spanish.
Cited by
-
Abnormalities in substance P neurokinin-1 receptor binding in key brainstem nuclei in sudden infant death syndrome related to prematurity and sex.PLoS One. 2017 Sep 20;12(9):e0184958. doi: 10.1371/journal.pone.0184958. eCollection 2017. PLoS One. 2017. PMID: 28931039 Free PMC article.
-
Investigating genetic variants in microRNA regulators of Neurokinin-1 receptor in sudden infant death syndrome.Acta Paediatr. 2023 Feb;112(2):273-276. doi: 10.1111/apa.16580. Epub 2022 Nov 2. Acta Paediatr. 2023. PMID: 36271909 Free PMC article.
-
Chronic Intermittent Hypoxia Differentially Impacts Different States of Inspiratory Activity at the Level of the preBötzinger Complex.Front Physiol. 2017 Aug 28;8:571. doi: 10.3389/fphys.2017.00571. eCollection 2017. Front Physiol. 2017. PMID: 28936176 Free PMC article.
-
The physiological determinants of sudden infant death syndrome.Respir Physiol Neurobiol. 2013 Nov 1;189(2):288-300. doi: 10.1016/j.resp.2013.05.032. Epub 2013 Jun 2. Respir Physiol Neurobiol. 2013. PMID: 23735486 Free PMC article. Review.
-
Respiratory frequency plasticity during development.Respir Physiol Neurobiol. 2019 Aug;266:54-65. doi: 10.1016/j.resp.2019.04.014. Epub 2019 May 3. Respir Physiol Neurobiol. 2019. PMID: 31055188 Free PMC article. Review.
References
-
- Bramlett HM, Dietrich WD (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 24: 133–150. - PubMed
-
- Young T (1993) Analytic epidemiology studies of sleep disordered breathing–what explains the gender difference in sleep disordered breathing? Sleep 16: S1–2. - PubMed
-
- Chervin RD, Archbold KH, Dillon JE, Panahi P, Pituch KJ, et al. (2002) Inattention, hyperactivity, and symptoms of sleep-disordered breathing. Pediatrics 109: 449–456. - PubMed
-
- Webber MP, Carpiniello KE, Oruwariye T, Appel DK (2002) Prevalence of asthma and asthma-like symptoms in inner-city elementary schoolchildren. Pediatr Pulmonol 34: 105–111. - PubMed
-
- Sanfilippo-Cohn B, Lai S, Zhan G, Fenik P, Pratico D, et al. (2006) Sex differences in susceptibility to oxidative injury and sleepiness from intermittent hypoxia. Sleep 29: 152–159. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

