Induced collagen cross-links enhance cartilage integration
- PMID: 23593295
- PMCID: PMC3617163
- DOI: 10.1371/journal.pone.0060719
Induced collagen cross-links enhance cartilage integration
Abstract
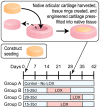
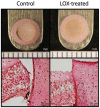
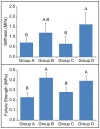
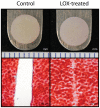
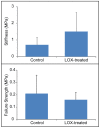
Articular cartilage does not integrate due primarily to a scarcity of cross-links and viable cells at the interface. The objective of this study was to test the hypothesis that lysyl-oxidase, a metalloenzyme that forms collagen cross-links, would be effective in improving integration between native-to-native, as well as tissue engineered-to-native cartilage surfaces. To examine these hypotheses, engineered cartilage constructs, synthesized via the self-assembling process, as well as native cartilage, were implanted into native cartilage rings and treated with lysyl-oxidase for varying amounts of time. For both groups, lysyl-oxidase application resulted in greater apparent stiffness across the cartilage interface 2-2.2 times greater than control. The construct-to-native lysyl-oxidase group also exhibited a statistically significant increase in the apparent strength, here defined as the highest observed peak stress during tensile testing. Histology indicated a narrowing gap at the cartilage interface in lysyl-oxidase treated groups, though this alone is not sufficient to indicate annealing. However, when the morphological and mechanical data are taken together, the longer the duration of lysyl-oxidase treatment, the more integrated the interface appeared. Though further data are needed to confirm the mechanism of action, the enhancement of integration may be due to lysyl-oxidase-induced pyridinoline cross-links. This study demonstrates that lysyl-oxidase is a potent agent for enhancing integration between both native-to-native and native-to-engineered cartilages. The fact that interfacial strength increased manifold suggests that cross-linking agents should play a significant role in solving the difficult problem of cartilage integration. Future studies must examine dose, dosing regimen, and cellular responses to lysyl-oxidase to optimize its application.
Conflict of interest statement
Figures
References
-
- Athanasiou KA, Darling EM, Hu JC (2009) Articular cartilage tissue engineering; Athanasiou KA, editor. Electronic reference: Morgan & Claypool.
-
- Smith GD, Knutsen G, Richardson JB (2005) A clinical review of cartilage repair techniques. J Bone Joint Surg Br 87: 445–449. - PubMed
-
- Wang W, Li B, Li Y, Jiang Y, Ouyang H, et al. (2010) In vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials 31: 5953–5965. - PubMed
-
- Kon E, Delcogliano M, Filardo G, Fini M, Giavaresi G, et al. (2010) Orderly osteochondral regeneration in a sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res 28: 116–124. - PubMed
-
- St-Pierre JP, Gan L, Wang J, Pilliar RM, Grynpas MD, et al. (2012) The incorporation of a zone of calcified cartilage improves the interfacial shear strength between in vitro-formed cartilage and the underlying substrate. Acta Biomater 8: 1603–1615. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

