Structural and functional role of INI1 and LEDGF in the HIV-1 preintegration complex
- PMID: 23593299
- PMCID: PMC3623958
- DOI: 10.1371/journal.pone.0060734
Structural and functional role of INI1 and LEDGF in the HIV-1 preintegration complex
Abstract
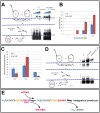
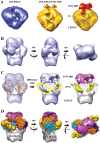
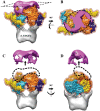
Integration of the HIV-1 cDNA into the human genome is catalyzed by the viral integrase (IN) protein. Several studies have shown the importance of cellular cofactors that interact with integrase and affect viral integration and infectivity. In this study, we produced a stable complex between HIV-1 integrase, viral U5 DNA, the cellular cofactor LEDGF/p75 and the integrase binding domain of INI1 (INI1-IBD), a subunit of the SWI/SNF chromatin remodeling factor. The stoichiometry of the IN/LEDGF/INI1-IBD/DNA complex components was found to be 4/2/2/2 by mass spectrometry and Fluorescence Correlation Spectroscopy. Functional assays showed that INI1-IBD inhibits the 3' processing reaction but does not interfere with specific viral DNA binding. Integration assays demonstrate that INI1-IBD decreases the amount of integration events but inhibits by-product formation such as donor/donor or linear full site integration molecules. Cryo-electron microscopy locates INI1-IBD within the cellular DNA binding site of the IN/LEDGF complex, constraining the highly flexible integrase in a stable conformation. Taken together, our results suggest that INI1 could stabilize the PIC in the host cell, by maintaining integrase in a stable constrained conformation which prevents non-specific interactions and auto integration on the route to its integration site within nucleosomes, while LEDGF organizes and stabilizes an active integrase tetramer suitable for specific vDNA integration. Moreover, our results provide the basis for a novel type of integrase inhibitor (conformational inhibitor) representing a potential new strategy for use in human therapy.
Conflict of interest statement
Figures


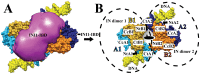

References
-
- Chiu TK, Davies DR (2004) Structure and function of HIV-1 integrase. CurrTopMedChem 4: 965–977. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

