Virologic response to tipranavir-ritonavir or darunavir-ritonavir based regimens in antiretroviral therapy experienced HIV-1 patients: a meta-analysis and meta-regression of randomized controlled clinical trials
- PMID: 23593314
- PMCID: PMC3617230
- DOI: 10.1371/journal.pone.0060814
Virologic response to tipranavir-ritonavir or darunavir-ritonavir based regimens in antiretroviral therapy experienced HIV-1 patients: a meta-analysis and meta-regression of randomized controlled clinical trials
Abstract
Background: The development of tipranavir and darunavir, second generation non-peptidic HIV protease inhibitors, with marked improved resistance profiles, has opened a new perspective on the treatment of antiretroviral therapy (ART) experienced HIV patients with poor viral load control. The aim of this study was to determine the virologic response in ART experienced patients to tipranavir-ritonavir and darunavir-ritonavir based regimens.
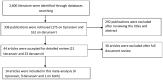
Methods and findings: A computer based literature search was conducted in the databases of HINARI (Health InterNetwork Access to Research Initiative), Medline and Cochrane library. Meta-analysis was performed by including randomized controlled studies that were conducted in ART experienced patients with plasma viral load above 1,000 copies HIV RNA/ml. The odds ratios and 95% confidence intervals (CI) for viral loads of <50 copies and <400 copies HIV RNA/ml at the end of the intervention were determined by the random effects model. Meta-regression, sensitivity analysis and funnel plots were done. The number of HIV-1 patients who were on either a tipranavir-ritonavir or darunavir-ritonavir based regimen and achieved viral load less than 50 copies HIV RNA/ml was significantly higher (overall OR = 3.4; 95% CI, 2.61-4.52) than the number of HIV-1 patients who were on investigator selected boosted comparator HIV-1 protease inhibitors (CPIs-ritonavir). Similarly, the number of patients with viral load less than 400 copies HIV RNA/ml was significantly higher in either the tipranavir-ritonavir or darunavir-ritonavir based regimen treated group (overall OR = 3.0; 95% CI, 2.15-4.11). Meta-regression showed that the viral load reduction was independent of baseline viral load, baseline CD4 count and duration of tipranavir-ritonavir or darunavir-ritonavir based regimen.
Conclusions: Tipranavir and darunavir based regimens were more effective in patients who were ART experienced and had poor viral load control. Further studies are required to determine their consistent viral load suppression effect as the duration of treatment is more prolonged.
Conflict of interest statement
Figures



Similar articles
-
Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials.Lancet. 2006 Aug 5;368(9534):466-75. doi: 10.1016/S0140-6736(06)69154-X. Lancet. 2006. PMID: 16890833
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
-
Relative antiviral efficacy of ritonavir-boosted darunavir and ritonavir-boosted tipranavir vs. control protease inhibitor in the POWER and RESIST trials.HIV Med. 2007 May;8(4):259-64. doi: 10.1111/j.1468-1293.2007.00465.x. HIV Med. 2007. PMID: 17461854 Clinical Trial.
-
Pharmacokinetic characterization of three doses of tipranavir boosted with ritonavir on highly active antiretroviral therapy in treatment-experienced HIV-1 patients.HIV Clin Trials. 2010 Jan-Feb;11(1):28-38. doi: 10.1310/hct1101-28. HIV Clin Trials. 2010. PMID: 20400409 Clinical Trial.
-
Tipranavir: PNU 140690, tipranivir.Drugs R D. 2006;7(1):55-62. doi: 10.2165/00126839-200607010-00005. Drugs R D. 2006. PMID: 16620137 Review.
Cited by
-
Virologic Effectiveness of Abacavir/Lamivudine with Darunavir/Ritonavir Versus Other Protease Inhibitors in Treatment-Experienced HIV-Infected Patients in Clinical Practice.Clin Drug Investig. 2017 Jan;37(1):51-60. doi: 10.1007/s40261-016-0456-1. Clin Drug Investig. 2017. PMID: 27587070 Free PMC article.
-
The role of mutations at codons 32, 47, 54, and 90 in HIV-1 protease flap dynamics.Discoveries (Craiova). 2014 Dec 31;2(4):e27. doi: 10.15190/d.2014.19. Discoveries (Craiova). 2014. PMID: 32309558 Free PMC article.
-
Effectiveness, durability, and safety of darunavir/ritonavir in HIV-1-infected patients in routine clinical practice in Italy: a postauthorization noninterventional study.Drug Des Devel Ther. 2016 May 6;10:1589-603. doi: 10.2147/DDDT.S104875. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27226708 Free PMC article.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) (2012) Together we will end AIDS. Available: http://www.google.com.et/url. Accessed 2012 Oct.
-
- Phillips AN, Dunn D, Sabin C, Pozniak A, Matthias R, et al. (2005) Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS 19: 487–94. - PubMed
-
- Kozal M (2004) Cross-resistance patterns among HIV protease inhibitors. AIDS Patient Care ST 18(4): 199–208. - PubMed
-
- Lima VD, Gill VS, Yip B, Hogg RS, Montaner JSG, et al. (2008) Increased resilience to the development of drug resistance with modern boosted protease inhibitor– based highly active antiretroviral therapy. J Infect Dis 198: 51–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

