Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy
- PMID: 23593350
- PMCID: PMC3623942
- DOI: 10.1371/journal.pone.0060931
Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy
Abstract
Background/aim: Hypercaloric diet ingestion and sedentary lifestyle result in obesity. Metabolic syndrome is a cluster of clinical features secondary to obesity, considered as a pre-diabetic condition and recognized as an independent risk factor for cardiovascular diseases. To better understand the relationship between obesity, metabolic syndrome and cardiovascular disease as well as for the development of novel therapeutic strategies, animal models that reproduce the etiology, course and outcomes of these pathologies are required. The aim of this work was to characterize the long-term effects of high-fat diet-induced obesity on the mice cardiovascular system, in order to make available a new animal model for diabetic cardiomyopathy.
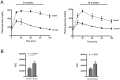
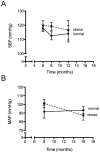
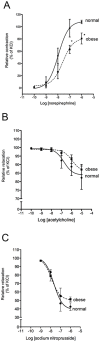
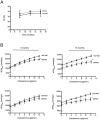
Methods/results: Male C57BL/6 mice were fed with a standardized high-fat diet (obese) or regular diet (normal) for 16 months. Metabolic syndrome was evaluated testing plasma glucose, triglycerides, cholesterol, insulin, and glucose tolerance. Arterial pressure was measured using a sphygmomanometer (non invasive method) and by hemodynamic parameters (invasive method). Cardiac anatomy was described based on echocardiography and histological studies. Cardiac function was assessed by cardiac catheterization under a stress test. Cardiac remodelling and metabolic biomarkers were assessed by RT-qPCR and immunoblotting. As of month eight, the obese mice were overweight, hyperglycaemic, insulin resistant, hyperinsulinemic and hypercholesterolemic. At month 16, they also presented normal arterial pressure but altered vascular reactivity (vasoconstriction), and cardiac contractility reserve reduction, heart mass increase, cardiomyocyte hypertrophy, cardiac fibrosis, and heart metabolic compensations. By contrast, the normal mice remained healthy throughout the study.
Conclusions: Mice fed with a high-fat diet for prolonged time recapitulates the etiology, course and outcomes of the early phases of human diabetic cardiomyopathy.
Conflict of interest statement
Figures

Similar articles
-
Functional resilience of C57BL/6J mouse heart to dietary fat overload.Am J Physiol Heart Circ Physiol. 2021 Nov 1;321(5):H850-H864. doi: 10.1152/ajpheart.00419.2021. Epub 2021 Sep 3. Am J Physiol Heart Circ Physiol. 2021. PMID: 34477461 Free PMC article.
-
Administration of granulocyte-colony stimulating factor accompanied with a balanced diet improves cardiac function alterations induced by high fat diet in mice.BMC Cardiovasc Disord. 2015 Dec 3;15:162. doi: 10.1186/s12872-015-0154-6. BMC Cardiovasc Disord. 2015. PMID: 26631050 Free PMC article.
-
High-Fat Diet-Induced Diabetic Cardiomyopathy in Female Zebrafish: Cardiac Pathology and Functional Decline Mediated by Type 2 Diabetes.Nutrients. 2025 Jul 2;17(13):2209. doi: 10.3390/nu17132209. Nutrients. 2025. PMID: 40647313 Free PMC article.
-
Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies.Vasc Health Risk Manag. 2010 Oct 21;6:883-903. doi: 10.2147/VHRM.S11681. Vasc Health Risk Manag. 2010. PMID: 21057575 Free PMC article. Review.
-
Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging.Nat Rev Cardiol. 2021 Apr;18(4):291-304. doi: 10.1038/s41569-020-00465-5. Epub 2020 Nov 13. Nat Rev Cardiol. 2021. PMID: 33188304 Review.
Cited by
-
Cardiometabolic Changes in Sirtuin1-Heterozygous Mice on High-Fat Diet and Melatonin Supplementation.Int J Mol Sci. 2024 Jan 10;25(2):860. doi: 10.3390/ijms25020860. Int J Mol Sci. 2024. PMID: 38255934 Free PMC article.
-
Nuclear-mitochondrial communication involving miR-181c plays an important role in cardiac dysfunction during obesity.J Mol Cell Cardiol. 2020 Jul;144:87-96. doi: 10.1016/j.yjmcc.2020.05.009. Epub 2020 May 19. J Mol Cell Cardiol. 2020. PMID: 32442661 Free PMC article.
-
Role of autophagy in a model of obesity: A long‑term high fat diet induces cardiac dysfunction.Mol Med Rep. 2018 Sep;18(3):3251-3261. doi: 10.3892/mmr.2018.9301. Epub 2018 Jul 23. Mol Med Rep. 2018. PMID: 30066870 Free PMC article.
-
Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities.J Mol Cell Cardiol. 2016 Jan;90:84-93. doi: 10.1016/j.yjmcc.2015.12.011. Epub 2015 Dec 15. J Mol Cell Cardiol. 2016. PMID: 26705059 Free PMC article. Review.
-
Loss of the melanocortin-4 receptor in mice causes dilated cardiomyopathy.Elife. 2017 Aug 22;6:e28118. doi: 10.7554/eLife.28118. Elife. 2017. PMID: 28829041 Free PMC article.
References
-
- Reaven GM, Chen YD (1988) Role of insulin in regulation of lipoprotein metabolism in diabetes. Diabetes/metabolism reviews 4: 639–652. - PubMed
-
- Bonora E, Kiechl S, Willeit J, Oberhollenzer F, Egger G, et al. (2003) Metabolic syndrome: epidemiology and more extensive phenotypic description. Cross-sectional data from the Bruneck Study. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 27: 1283–1289. - PubMed
-
- Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, et al. (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes care 24: 683–689. - PubMed
-
- Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, et al. (2002) The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA : the journal of the American Medical Association 288: 2709–2716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

