Zoledronic acid restores doxorubicin chemosensitivity and immunogenic cell death in multidrug-resistant human cancer cells
- PMID: 23593363
- PMCID: PMC3625183
- DOI: 10.1371/journal.pone.0060975
Zoledronic acid restores doxorubicin chemosensitivity and immunogenic cell death in multidrug-resistant human cancer cells
Retraction in
-
Retraction: Zoledronic Acid Restores Doxorubicin Chemosensitivity and Immunogenic Cell Death in Multidrug-Resistant Human Cancer Cells.PLoS One. 2020 Dec 15;15(12):e0244194. doi: 10.1371/journal.pone.0244194. eCollection 2020. PLoS One. 2020. PMID: 33320892 Free PMC article. No abstract available.
Abstract
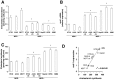
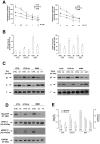
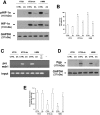
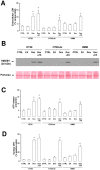
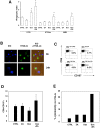
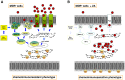
Durable tumor cell eradication by chemotherapy is challenged by the development of multidrug-resistance (MDR) and the failure to induce immunogenic cell death. The aim of this work was to investigate whether MDR and immunogenic cell death share a common biochemical pathway eventually amenable to therapeutic intervention. We found that mevalonate pathway activity, Ras and RhoA protein isoprenylation, Ras- and RhoA-downstream signalling pathway activities, Hypoxia Inducible Factor-1alpha activation were significantly higher in MDR+ compared with MDR- human cancer cells, leading to increased P-glycoprotein expression, and protection from doxorubicin-induced cytotoxicity and immunogenic cell death. Zoledronic acid, a potent aminobisphosphonate targeting the mevalonate pathway, interrupted Ras- and RhoA-dependent downstream signalling pathways, abrogated the Hypoxia Inducible Factor-1alpha-driven P-glycoprotein expression, and restored doxorubicin-induced cytotoxicity and immunogenic cell death in MDR+ cells. Immunogenic cell death recovery was documented by the ability of dendritic cells to phagocytise MDR+ cells treated with zoledronic acid plus doxorubicin, and to recruit anti-tumor cytotoxic CD8+ T lymphocytes. These data indicate that MDR+ cells have an hyper-active mevalonate pathway which is targetable with zoledronic acid to antagonize their ability to withstand chemotherapy-induced cytotoxicity and escape immunogenic cell death.
Conflict of interest statement
Figures

References
-
- Swanson KM, Hohl RJ (2006) Anti-Cancer Therapy: Targeting the Mevalonate Pathway. Curr Cancer Drug Targets 6: 15–37. - PubMed
-
- Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2: 48–58. - PubMed
-
- Troost J, Lindenmaie J, Haefeli WE, Weiss J (2004) Modulation of cellular cholesterol alters P-glycoprotein activity in multidrug-resistant cells. Mol Pharmacol 66: 1332–1339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

