Molecular characterization of branchial aquaporin 1aa and effects of seawater acclimation, emersion or ammonia exposure on its mRNA expression in the gills, gut, kidney and skin of the freshwater climbing perch, Anabas testudineus
- PMID: 23593418
- PMCID: PMC3621907
- DOI: 10.1371/journal.pone.0061163
Molecular characterization of branchial aquaporin 1aa and effects of seawater acclimation, emersion or ammonia exposure on its mRNA expression in the gills, gut, kidney and skin of the freshwater climbing perch, Anabas testudineus
Abstract
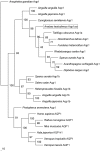
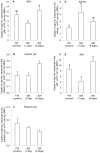
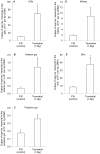
We obtained a full cDNA coding sequence of aquaporin 1aa (aqp1aa) from the gills of the freshwater climbing perch, Anabas testudineus, which had the highest expression in the gills and skin, suggesting an important role of Aqp1aa in these organs. Since seawater acclimation had no significant effects on the branchial and intestinal aqp1aa mRNA expression, and since the mRNA expression of aqp1aa in the gut was extremely low, it can be deduced that Aqp1aa, despite being a water channel, did not play a significant osmoregulatory role in A. testudineus. However, terrestrial exposure led to significant increases in the mRNA expression of aqp1aa in the gills and skin of A. testudineus. Since terrestrial exposure would lead to evaporative water loss, these results further support the proposition that Aqp1aa did not function predominantly for the permeation of water through the gills and skin. Rather, increased aqp1aa mRNA expression might be necessary to facilitate increased ammonia excretion during emersion, because A. testudineus is known to utilize amino acids as energy sources for locomotor activity with increased ammonia production on land. Furthermore, ammonia exposure resulted in significant decreases in mRNA expression of aqp1aa in the gills and skin of A. testudineus, presumably to reduce ammonia influx during ammonia loading. This corroborates previous reports on AQP1 being able to facilitate ammonia permeation. However, a molecular characterization of Aqp1aa from A. testudineus revealed that its intrinsic aquapore might not facilitate NH3 transport. Hence, ammonia probably permeated the central fifth pore of the Aqp1aa tetramer as suggested previously. Taken together, our results indicate that Aqp1aa might have a greater physiological role in ammonia excretion than in osmoregulation in A. testudineus.
Conflict of interest statement
Figures



Similar articles
-
Both seawater acclimation and environmental ammonia exposure lead to increases in mRNA expression and protein abundance of Na⁺:K⁺:2Cl⁻ cotransporter in the gills of the climbing perch, Anabas testudineus.J Comp Physiol B. 2012 May;182(4):491-506. doi: 10.1007/s00360-011-0634-7. Epub 2011 Dec 18. J Comp Physiol B. 2012. PMID: 22179410
-
Cystic fibrosis transmembrane conductance regulator in the gills of the climbing perch, Anabas testudineus, is involved in both hypoosmotic regulation during seawater acclimation and active ammonia excretion during ammonia exposure.J Comp Physiol B. 2012 Aug;182(6):793-812. doi: 10.1007/s00360-012-0664-9. Epub 2012 Apr 22. J Comp Physiol B. 2012. PMID: 22526263
-
Roles of three branchial Na(+)-K(+)-ATPase α-subunit isoforms in freshwater adaptation, seawater acclimation, and active ammonia excretion in Anabas testudineus.Am J Physiol Regul Integr Comp Physiol. 2012 Jul 1;303(1):R112-25. doi: 10.1152/ajpregu.00618.2011. Epub 2012 May 23. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22621969
-
Na+/H+ Exchanger 3 Is Expressed in Two Distinct Types of Ionocyte, and Probably Augments Ammonia Excretion in One of Them, in the Gills of the Climbing Perch Exposed to Seawater.Front Physiol. 2017 Nov 2;8:880. doi: 10.3389/fphys.2017.00880. eCollection 2017. Front Physiol. 2017. PMID: 29209224 Free PMC article.
-
A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins.J Exp Biol. 2009 Aug;212(Pt 15):2303-12. doi: 10.1242/jeb.023085. J Exp Biol. 2009. PMID: 19617422 Review.
Cited by
-
Aquaporin 1 Is Involved in Acid Secretion by Ionocytes of Zebrafish Embryos through Facilitating CO2 Transport.PLoS One. 2015 Aug 19;10(8):e0136440. doi: 10.1371/journal.pone.0136440. eCollection 2015. PLoS One. 2015. PMID: 26287615 Free PMC article.
-
High-resolution structure of a fish aquaporin reveals a novel extracellular fold.Life Sci Alliance. 2022 Oct 13;5(12):e202201491. doi: 10.26508/lsa.202201491. Life Sci Alliance. 2022. PMID: 36229063 Free PMC article.
-
Differential Expression and Localization of Branchial AQP1 and AQP3 in Japanese Medaka (Oryzias latipes).Cells. 2019 May 8;8(5):422. doi: 10.3390/cells8050422. Cells. 2019. PMID: 31072010 Free PMC article.
-
Ion Transporters and Osmoregulation in the Kidney of Teleost Fishes as a Function of Salinity.Front Physiol. 2021 Apr 20;12:664588. doi: 10.3389/fphys.2021.664588. eCollection 2021. Front Physiol. 2021. PMID: 33967835 Free PMC article. Review.
-
Molecular Characterization of Aquaporin 1 and Aquaporin 3 from the Gills of the African Lungfish, Protopterus annectens, and Changes in Their Branchial mRNA Expression Levels and Protein Abundance during Three Phases of Aestivation.Front Physiol. 2016 Nov 10;7:532. doi: 10.3389/fphys.2016.00532. eCollection 2016. Front Physiol. 2016. PMID: 27891097 Free PMC article.
References
-
- King SL, Agre P (2001) Water Channels. Baltimore, MD: John Wiley & Sons, Ltd.
-
- Shi L, Skach W, Verkman A (1994) Functional independence of monomeric CHIP28 water channels revealed by expression of wild-type mutant heterodimers. J Biol Chem 269: 10417–10422. - PubMed
-
- Gonen T, Walz T (2006) The structure of aquaporins. Q Rev Biophys 39: 361–396. - PubMed
-
- Verkman AS, Mitra AK (2000) Structure and function of aquaporin water channels. Am J Physiol Renal Physiol 278: F13–F28. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

