Variable VlsE is critical for host reinfection by the Lyme disease spirochete
- PMID: 23593438
- PMCID: PMC3620393
- DOI: 10.1371/journal.pone.0061226
Variable VlsE is critical for host reinfection by the Lyme disease spirochete
Abstract
Many pathogens make use of antigenic variation as a way to evade the host immune response. A key mechanism for immune evasion and persistent infection by the Lyme disease spirochete, Borrelia burgdorferi, is antigenic variation of the VlsE surface protein. Recombination results in changes in the VlsE surface protein that prevent recognition by VlsE-specific antibodies in the infected host. Despite the presence of a substantial number of additional proteins residing on the bacterial surface, VlsE is the only known antigen that exhibits ongoing variation of its surface epitopes. This suggests that B. burgdorferi may utilize a VlsE-mediated system for immune avoidance of its surface antigens. To address this, the requirement of VlsE for host reinfection by the Lyme disease pathogen was investigated. Host-adapted wild type and VlsE mutant spirochetes were used to reinfect immunocompetent mice that had naturally cleared an infection with a VlsE-deficient clone. Our results demonstrate that variable VlsE is necessary for reinfection by B. burgdorferi, and this ability is directly related to evasion of the host antibody response. Moreover, the data presented here raise the possibility that VlsE prevents recognition of B. burgdorferi surface antigens from host antibodies. Overall, our findings represent a significant advance in our knowledge of immune evasion by B. burgdorferi, and provide insight to the possible mechanisms involved in VlsE-mediated immune avoidance.
Conflict of interest statement
Figures


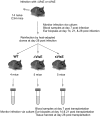


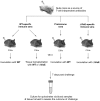
Similar articles
-
Changing of the guard: How the Lyme disease spirochete subverts the host immune response.J Biol Chem. 2020 Jan 10;295(2):301-313. doi: 10.1074/jbc.REV119.008583. Epub 2019 Nov 21. J Biol Chem. 2020. PMID: 31753921 Free PMC article. Review.
-
Identification of Surface Epitopes Associated with Protection against Highly Immune-Evasive VlsE-Expressing Lyme Disease Spirochetes.Infect Immun. 2018 Jul 23;86(8):e00182-18. doi: 10.1128/IAI.00182-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29866906 Free PMC article.
-
YebC regulates variable surface antigen VlsE expression and is required for host immune evasion in Borrelia burgdorferi.PLoS Pathog. 2020 Oct 13;16(10):e1008953. doi: 10.1371/journal.ppat.1008953. eCollection 2020 Oct. PLoS Pathog. 2020. PMID: 33048986 Free PMC article.
-
The Borrelia burgdorferi VlsE Lipoprotein Prevents Antibody Binding to an Arthritis-Related Surface Antigen.Cell Rep. 2020 Mar 17;30(11):3663-3670.e5. doi: 10.1016/j.celrep.2020.02.081. Cell Rep. 2020. PMID: 32187539 Free PMC article.
-
vls Antigenic Variation Systems of Lyme Disease Borrelia: Eluding Host Immunity through both Random, Segmental Gene Conversion and Framework Heterogeneity.Microbiol Spectr. 2014 Dec;2(6):10.1128/microbiolspec.MDNA3-0038-2014. doi: 10.1128/microbiolspec.MDNA3-0038-2014. Microbiol Spectr. 2014. PMID: 26104445 Free PMC article. Review.
Cited by
-
Subversion of the immune response of human pathogenic spirochetes.J Clin Lab Anal. 2022 May;36(5):e24414. doi: 10.1002/jcla.24414. Epub 2022 Apr 11. J Clin Lab Anal. 2022. PMID: 35403248 Free PMC article. Review.
-
Polymorphism of 41 kD Flagellin Gene and Its Human B-Cell Epitope in Borrelia burgdorferi Strains of China.Biomed Res Int. 2016;2016:1327320. doi: 10.1155/2016/1327320. Epub 2016 Nov 15. Biomed Res Int. 2016. PMID: 28042565 Free PMC article.
-
Changing of the guard: How the Lyme disease spirochete subverts the host immune response.J Biol Chem. 2020 Jan 10;295(2):301-313. doi: 10.1074/jbc.REV119.008583. Epub 2019 Nov 21. J Biol Chem. 2020. PMID: 31753921 Free PMC article. Review.
-
Role of the VlsE Lipoprotein in Immune Avoidance by the Lyme Disease Spirochete Borrelia burgdorferi.For Immunopathol Dis Therap. 2016;7(3-4):191-204. doi: 10.1615/ForumImmunDisTher.2017019625. For Immunopathol Dis Therap. 2016. PMID: 29876140 Free PMC article.
-
Correlation between antigenicity and variability in the vls antigenic variation system of Borrelia burgdorferi.Microbes Infect. 2017 Apr-May;19(4-5):267-276. doi: 10.1016/j.micinf.2017.01.001. Epub 2017 Jan 10. Microbes Infect. 2017. PMID: 28087455 Free PMC article.
References
-
- Barbour AG (2001) Borrelia: a diverse and ubiquitous genus of tick-borne pathogens. In: Scheld MW, Craig WA, Hughes JM, editors. Emerging Infections 5. Washington, D.C.: American Society for Microbiology. pp. 153–173.
-
- Steere AC (2001) Lyme disease. N Engl J Med 345: 115–125. - PubMed
-
- Krause PJ, Foley DT, Burke GS, Christianson D, Closter L, et al. (2006) Reinfection and relapse in early Lyme disease. Am J Trop Med Hyg 75: 1090–1094. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

