Effects of meat cooking, and of ingested amount, on protein digestion speed and entry of residual proteins into the colon: a study in minipigs
- PMID: 23593443
- PMCID: PMC3625175
- DOI: 10.1371/journal.pone.0061252
Effects of meat cooking, and of ingested amount, on protein digestion speed and entry of residual proteins into the colon: a study in minipigs
Abstract
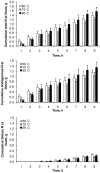
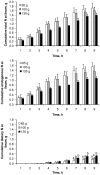
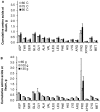
The speed of protein digestion impacts on postprandial protein anabolism. After exercise or in the elderly, fast proteins stimulate protein synthesis more efficiently than slow proteins. It has been shown that meat might be a source of fast proteins. However, cooking temperature, acting on the macrostructure and microstructure of the meat could affect both the speed, and efficiency, of protein digestion. This study aims to evaluate, in vivo, the effect of meat cooking on digestion parameters, in the context of a complete meal. Six minipigs fitted with an ileal cannula and an arterial catheter were used. In order to measure the true ileal digestibility, tested meat was obtained from a calf, the muscle proteins of which were intrinsically labelled with (15)N-amino acids. Three cooking temperatures (60, 75 and 95°C; core temperature for 30 min), and three levels of intake (1, 1.45, and 1.90 g protein/kg body weight) were tested. Following meat ingestion, ileal digesta and arterial blood were collected over a 9-h period. The speed of digestion, evaluated from the kinetics of amino acid appearance in blood within the first 3 h, was greater for the cooking temperature of 75°C, than for 60 or 95°C. The true ileal digestibility, which averaged 95%, was not affected by cooking temperature or by the level of meat intake. The amino acid composition of the digesta flowing at the ileum was not affected by cooking temperature. These results show that cooking temperature can modulate the speed of meat protein digestion, without affecting the efficiency of the small intestinal digestion, and consequently the entry of meat protein residues into the colon.
Conflict of interest statement
Figures

References
-
- Bos C, Juillet B, Fouillet H, Turlan L, Daré S, et al. (2005) Postprandial metabolic utilization of wheat protein in humans. Am J Clin Nutr 81: 87–94. - PubMed
-
- Bos C, Airinei G, Mariotti F, Benamouzig R, Bérot S, et al. (2007) The poor digestibility of rapeseed protein is balanced by its very high metabolic utilization in humans. J Nutr 137: 594–600. - PubMed
-
- Gaudichon C, Bos C, Morens C, Petzke KJ, Mariotti F, et al. (2002) Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterol 123: 50–59. - PubMed
-
- Mariotti F, Mahé S, Benamouzig R, Luengo C, Daré S, et al. (1999) Nutritional value of [15N]-soy protein isolate assessed from ileal digestibility and postprandial protein utilization in humans. J Nutr 129: 1992–1997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

