Evaluation of an intranasal virosomal vaccine against respiratory syncytial virus in mice: effect of TLR2 and NOD2 ligands on induction of systemic and mucosal immune responses
- PMID: 23593453
- PMCID: PMC3620164
- DOI: 10.1371/journal.pone.0061287
Evaluation of an intranasal virosomal vaccine against respiratory syncytial virus in mice: effect of TLR2 and NOD2 ligands on induction of systemic and mucosal immune responses
Abstract
Introduction: RSV infection remains a serious threat to newborns and the elderly. Currently, there is no vaccine available to prevent RSV infection. A mucosal RSV vaccine would be attractive as it could induce mucosal as well as systemic antibodies, capable of protecting both the upper and lower respiratory tract. Previously, we reported on a virosomal RSV vaccine for intramuscular injection with intrinsic adjuvant properties mediated by an incorporated lipophilic Toll-like receptor 2 (TLR2) ligand. However, it has not been investigated whether this virosomal RSV vaccine candidate would be suitable for use in mucosal immunization strategies and if additional incorporation of other innate receptor ligands, like NOD2-ligand, could further enhance the immunogenicity and protective efficacy of the vaccine.
Objective: To explore if intranasal (IN) immunization with a virosomal RSV vaccine, supplemented with TLR2 and/or NOD2-ligands, is an effective strategy to induce RSV-specific immunity.
Methods: We produced RSV-virosomes carrying TLR2 (Pam3CSK4) and/or NOD2 (L18-MDP) ligands. We tested the immunopotentiating properties of these virosomes in vitro, using TLR2- and/or NOD2-ligand-responsive murine and human cell lines, and in vivo by assessing induction of protective antibody and cellular responses upon IN immunization of BALB/c mice.
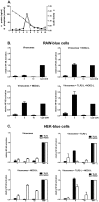
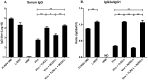
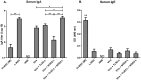
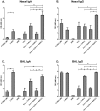
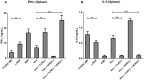
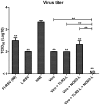
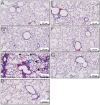
Results: Incorporation of Pam3CSK4 and/or L18-MDP potentiates the capacity of virosomes to activate (antigen-presenting) cells in vitro, as demonstrated by NF-κB induction. In vivo, incorporation of Pam3CSK4 in virosomes boosted serum IgG antibody responses and mucosal antibody responses after IN immunization. While L18-MDP alone was ineffective, incorporation of L18-MDP in Pam3CSK4-carrying virosomes further boosted mucosal antibody responses. Finally, IN immunization with adjuvanted virosomes, particularly Pam3CSK4/L18-MDP-adjuvanted-virosomes, protected mice against infection with RSV, without priming for enhanced disease.
Conclusion: Mucosal immunization with RSV-virosomes, supplemented with incorporated TLR2- and/or NOD2-ligands, represents a promising approach to induce effective and safe RSV-specific immunity.
Conflict of interest statement
Figures
Similar articles
-
Induction of mucosal and systemic immunity against respiratory syncytial virus by inactivated virus supplemented with TLR9 and NOD2 ligands.Vaccine. 2012 Jan 11;30(3):597-606. doi: 10.1016/j.vaccine.2011.11.054. Epub 2011 Nov 24. Vaccine. 2012. PMID: 22120195
-
Efficacy and safety of an intranasal virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice and cotton rats.Vaccine. 2013 Apr 19;31(17):2169-76. doi: 10.1016/j.vaccine.2013.02.043. Epub 2013 Mar 13. Vaccine. 2013. PMID: 23499594
-
Evaluation of dual pathogen recognition receptor agonists as adjuvants for respiratory syncytial virus - virus-like particles for pulmonary delivery.Front Immunol. 2025 Mar 17;16:1561297. doi: 10.3389/fimmu.2025.1561297. eCollection 2025. Front Immunol. 2025. PMID: 40176816 Free PMC article.
-
Targeting TLR2 for vaccine development.J Immunol Res. 2014;2014:619410. doi: 10.1155/2014/619410. Epub 2014 Jun 26. J Immunol Res. 2014. PMID: 25057505 Free PMC article. Review.
-
Advances in intranasal vaccine delivery: A promising non-invasive route of immunization.Vaccine. 2023 Jun 1;41(24):3589-3603. doi: 10.1016/j.vaccine.2023.05.011. Epub 2023 May 11. Vaccine. 2023. PMID: 37179163 Free PMC article. Review.
Cited by
-
Leukemia inhibitory factor protects the lung during respiratory syncytial viral infection.BMC Immunol. 2014 Oct 3;15:41. doi: 10.1186/s12865-014-0041-4. BMC Immunol. 2014. PMID: 25277705 Free PMC article.
-
Signaling via pattern recognition receptors NOD2 and TLR2 contributes to immunomodulatory control of lethal pneumovirus infection.Antiviral Res. 2016 Aug;132:131-40. doi: 10.1016/j.antiviral.2016.06.002. Epub 2016 Jun 14. Antiviral Res. 2016. PMID: 27312104 Free PMC article.
-
Nod2 is required for antigen-specific humoral responses against antigens orally delivered using a recombinant Lactobacillus vaccine platform.PLoS One. 2018 May 7;13(5):e0196950. doi: 10.1371/journal.pone.0196950. eCollection 2018. PLoS One. 2018. PMID: 29734365 Free PMC article.
-
Antigen-Sparing and Enhanced Efficacy of Multivalent Vaccines Adjuvanted with Immunopotentiators in Chickens.Front Microbiol. 2017 May 26;8:927. doi: 10.3389/fmicb.2017.00927. eCollection 2017. Front Microbiol. 2017. PMID: 28603519 Free PMC article.
-
Carbohydrate Immune Adjuvants in Subunit Vaccines.Pharmaceutics. 2020 Oct 14;12(10):965. doi: 10.3390/pharmaceutics12100965. Pharmaceutics. 2020. PMID: 33066594 Free PMC article. Review.
References
-
- van Drunen Littel-van den Hurk S, Mapletoft JW, Arsic N, Kovacs-Nolan J (2007) Immunopathology of RSV infection: prospects for developing vaccines without this complication. Rev Med Virol 17: 5–34. - PubMed
-
- Dowell SF, Anderson LJ, Gary HE, Erdman DD, Plouffe JF, et al. (1996) Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis 174: 456–462. - PubMed
-
- Hall CB, Walsh EE, Long CE, Schnabel KC (1991) Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 163: 693–698. - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, et al. (1969) Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89: 422–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

