Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons
- PMID: 23593498
- PMCID: PMC3625159
- DOI: 10.1371/journal.pone.0061787
Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons
Abstract
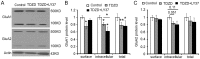
Group II metabotropic glutamate receptor (mGluR) agonists have emerged as potential treatment drugs for schizophrenia and other neurological disorders, whereas the mechanisms involved remain elusive. Here we examined the effects of LY379268 (LY37) on the expression and trafficking of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) receptor subunits GluA1 and GluA2 in prefrontal neurons. We show that LY37 significantly increased the surface and total expression of both GluA1 and GluA2 subunits in cultured prefrontal neurons and in vivo. This effect was mimicked by the selective mGluR2 agonist LY395756 and was blocked by mGluR2/3 antagonist LY341495. Moreover, we found that both GluA1 and GluA2 subunits were colocalized with PSD95 but not synapsin I, suggesting a postsynaptic localization. Consistently, treatment with LY37 significantly increased the amplitude, but not frequency, of miniature excitatory postsynaptic currents. Further, actinomycin-D blocked LY37's effects, suggesting a transcriptional regulation. In addition, application of glycogen synthase kinase-3beta (GSK-3β) inhibitor completely blocked LY37's effect on GluA2 surface expression, whereas GSK-3β inhibitor itself induced decreases in the surface and total protein levels of GluA1, but not GluA2 subunits. This suggests that GSK-3β differentially mediates GluA1 and GluA2 trafficking. Further, LY37 significantly increased the phosphorylation, but not total protein, of extracellular signal-regulated kinase 1/2 (ERK1/2). Neither ERK1/2 inhibitor PD98059 alone nor PD98059 combined with LY37 treatment induced changes in GluA1 or GluA2 surface expression or total protein levels. Our data thus suggest that mGluR2/3 agonist regulates postsynaptic AMPA receptors by affecting the synaptic trafficking of both GluA1 and GluA2 subunits and that the regulation is likely through ERK1/2 signaling in GluA1 and/or both ERK1/2 and GSK-3β signaling pathways in the GluA2 subunit.
Conflict of interest statement
Figures










References
-
- Lyon L, Burnet PW, Kew JN, Corti C, Rawlins JN, et al. (2011) Fractionation of spatial memory in GRM2/3 (mGlu2/mGlu3) double knockout mice reveals a role for group II metabotropic glutamate receptors at the interface between arousal and gognition. Neuropsychopharmacology 36: 2616–2628. - PMC - PubMed
-
- Fell MJ, McKinzie DL, Monn JA, Svensson KA (2012) Group II metabotropic glutamate receptor agonists and positive allosteric modulators as novel treatments for schizophrenia. Neuropharmacology 62: 1473–1483. - PubMed
-
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA (2008) The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol 22: 308–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

