Combined TLR7/8 and TLR9 ligands potentiate the activity of a Schistosoma japonicum DNA vaccine
- PMID: 23593527
- PMCID: PMC3617091
- DOI: 10.1371/journal.pntd.0002164
Combined TLR7/8 and TLR9 ligands potentiate the activity of a Schistosoma japonicum DNA vaccine
Abstract
Background: Toll-like receptor (TLR) ligands have been explored as vaccine adjuvants for tumor and virus immunotherapy, but few TLR ligands affecting schistosoma vaccines have been characterized. Previously, we developed a partially protective DNA vaccine encoding the 26-kDa glutathione S-transferase of Schistosoma japonicum (pVAX1-Sj26GST).
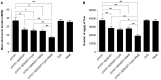
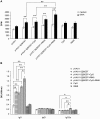
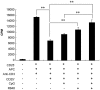
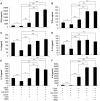
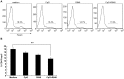
Methodology/principal findings: In this study, we evaluated a TLR7/8 ligand (R848) and a TLR9 ligand (CpG oligodeoxynucleotides, or CpG) as adjuvants for pVAX1-Sj26GST and assessed their effects on the immune system and protection against S. japonicum. We show that combining CpG and R848 with pVAX1-Sj26GST immunization significantly increases splenocyte proliferation and IgG and IgG2a levels, decreases CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg) frequency in vivo, and enhances protection against S. japonicum. CpG and R848 inhibited Treg-mediated immunosuppression, upregulated the production of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-4, IL-10, IL-2, and IL-6, and decreased Foxp3 expression in vitro, which may contribute to prevent Treg suppression and conversion during vaccination and allow expansion of antigen-specific T cells against pathogens.
Conclusions: Our data shows that selective TLR ligands can increase the protective efficacy of DNA vaccines against schistosomiasis, potentially through combined antagonism of Treg-mediated immunosuppression and conversion.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- King CH (2009) Toward the elimination of schistosomiasis. N Engl J Med 360: 106–109. - PubMed
-
- Doenhoff MJ, Cioli D, Utzinger J (2008) Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis 21: 659–667. - PubMed
-
- Abdul-Ghani R, Loutfy N, el-Sahn A, Hassan A (2009) Current chemotherapy arsenal for schistosomiasis mansoni: alternatives and challenges. Parasitol Res 104: 955–965. - PubMed
-
- Fenwick A, Webster JP, Bosque-Oliva E, Blair L, Fleming FM, et al. (2009) The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology 136: 1719–1730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

