Pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator, in the first-in-human study
- PMID: 23594176
- PMCID: PMC3845312
- DOI: 10.1111/bcp.12129
Pharmacokinetics and pharmacodynamics of ponesimod, a selective S1P1 receptor modulator, in the first-in-human study
Abstract
Aims: This study investigated the tolerability, safety, pharmacokinetics and pharmacodynamics of ponesimod, a novel oral selective sphingosine-1-phosphate (S1P1) receptor modulator in development for the treatment of auto-immune diseases.
Methods: This was a double-blind, placebo-controlled, ascending, single-dose study. Healthy male subjects received doses of 1-75 mg or placebo control.
Results: Ponesimod was well tolerated. Starting with a dose of 8 mg, transient asymptomatic reductions in heart rate were observed. Ponesimod pharmacokinetics were dose proportional. The median time to maximal concentration ranged from 2.0 to 4.0 h, and ponesimod was eliminated with a mean half-life varying between 21.7 and 33.4 h. Food had a minimal effect on ponesimod pharmacokinetics. Doses of ≥8 mg reduced total lymphocyte count in a dose-dependent manner. Lymphocyte counts returned to normal ranges within 96 h. A pharmacokinetic/pharmacodynamic model was developed that adequately described the observed effects of ponesimod on total lymphocyte counts.
Conclusions: Single doses of ponesimod up to and including 75 mg were well tolerated. The results of this ascending single-dose study indicate an immunomodulator potential for ponesimod and a pharmacokinetic/pharmacodynamic profile consistent with once-a-day dosing.
Keywords: S1P1 receptor modulator; pharmacodynamics; pharmacokinetics; ponesimod; safety and tolerability; total lymphocyte count.
© 2013 The British Pharmacological Society.
Figures
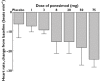

 , 1 mg;
, 1 mg;  , 3 mg;
, 3 mg;  , 8 mg;
, 8 mg;  , 20 mg;
, 20 mg;  , 50 mg;
, 50 mg;  , 75 mg
, 75 mg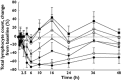
 , 1 mg;
, 1 mg;  , 3 mg;
, 3 mg;  , 8 mg;
, 8 mg;  , 20 mg;
, 20 mg;  , 50 mg;
, 50 mg;  , 75 mg;
, 75 mg;  , placebo
, placebo
 , placebo;
, placebo;  , 8 mg;
, 8 mg;  , 20 mg;
, 20 mg;  , 50 mg;
, 50 mg;  , 75 mg
, 75 mgReferences
-
- Tanaka T, Ebisuno Y, Kanemitsu N, Umemoto E, Yang BG, Jang MH, Miyasaka M. Molecular determinants controlling homeostatic recirculation and tissue-specific trafficking of lymphocytes. Int Arch Allergy Immunol. 2004;134:120–134. - PubMed
-
- Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. - PubMed
-
- Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. - PubMed
-
- Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. - PubMed
-
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

