A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care
- PMID: 23594434
- PMCID: PMC3720600
- DOI: 10.18632/oncotarget.969
A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care
Abstract
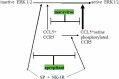
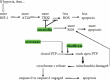
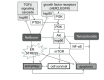
To improve prognosis in recurrent glioblastoma we developed a treatment protocol based on a combination of drugs not traditionally thought of as cytotoxic chemotherapy agents but that have a robust history of being well-tolerated and are already marketed and used for other non-cancer indications. Focus was on adding drugs which met these criteria: a) were pharmacologically well characterized, b) had low likelihood of adding to patient side effect burden, c) had evidence for interfering with a recognized, well-characterized growth promoting element of glioblastoma, and d) were coordinated, as an ensemble had reasonable likelihood of concerted activity against key biological features of glioblastoma growth. We found nine drugs meeting these criteria and propose adding them to continuous low dose temozolomide, a currently accepted treatment for relapsed glioblastoma, in patients with recurrent disease after primary treatment with the Stupp Protocol. The nine adjuvant drug regimen, Coordinated Undermining of Survival Paths, CUSP9, then are aprepitant, artesunate, auranofin, captopril, copper gluconate, disulfiram, ketoconazole, nelfinavir, sertraline, to be added to continuous low dose temozolomide. We discuss each drug in turn and the specific rationale for use- how each drug is expected to retard glioblastoma growth and undermine glioblastoma's compensatory mechanisms engaged during temozolomide treatment. The risks of pharmacological interactions and why we believe this drug mix will increase both quality of life and overall survival are reviewed.
Conflict of interest statement
Miguel Muñoz: U.S. Patent Trademark Office Application no. 20090012086: Use of nonpeptidic NK-1 receptor antagonists for the production of apoptosis in tumor cells. None of the other 27 authors have any conflict of interest.
Figures


References
-
- Stummer W, Meinel T, Ewelt C, Martus P, Jakobs O, Felsberg J, Reifenberger G. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol. 2012;108:89–97. doi: 10.1007/s11060-012-0798-3. - PMC - PubMed
-
- Conti A, Pontoriero A, Arpa D, Siragusa C, Tomasello C, Romanelli P, Cardali S, Granata F, De Renzis C, Tomasello F. Efficacy and toxicity of CyberKnife re-irradiation and “dose dense” temozolomide for recurrent gliomas. Acta Neurochir (Wien) 2012;154:203–9. doi: 10.1007/s00701-011-1184-1. - PubMed
-
- Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE, 2nd, Bailey L, Peters KB, Friedman HS, Vredenburgh JJ. Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer. 2012;118:1302–12. doi:10.1002/cncr.26381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

